Séminaire - 13/07/2023 - Shigeki KAWAI- Syntheses of Heteroatom-Substituted and Three-Dimensional Nanocarbon Materials on Surfaces
Invitation : Christian Loppacher (Département PHANO, Equipe NANO).
Diffusion : IM2NP, CINaM, Irphe, LP3 (via N. Sannier), Madirel (via P. Boulet), PIIM (via T. Angot), CPT (T. Martin), Fédération de Chimie (via S. Viel), CP2M
SEMINAIRE Jeudi 13 juillet 2023 à 11h00
Salle des séminaires de l'Im2np, campus de Saint-Jérôme, 1er étage Bâtiment Poincaré
Shigeki KAWAI
National Institute for Materials Science (NIMS), Tsukuba, Ibaraki 305-0047, Japan
University of Tsukuba, Tsukuba, Ibaraki, Japan
Syntheses of Heteroatom-Substituted and Three-Dimensional Nanocarbon Materials on Surfaces
The Since the first systematic on-surface covalent coupling of bromo-substituted porphyrins,[1] on-surface chemical synthesis has attracted tremendous interest of researchers.[2] In the reaction, appropriate precursor molecules are deposited on substrates by thermal evaporation under ultra-high vacuum and are subsequently linked to each other upon annealing treatment. The endeavor of exploiting on-surface chemical reactions is of central importance to develop various nanocarbon structures. Particularly, combining with bond-resolved scanning probe microscopy,[3] this field has been rapidly developed.
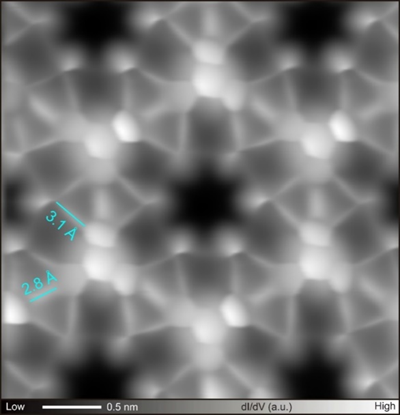
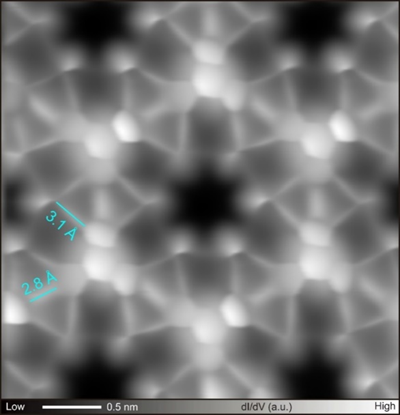
In this contribution, I will present our recent studies on on-surface chemistry with high-resolution scanning probe microscopy operating at low temperature under ultra-high vacuum. We synthesized three-dimensional graphene nanoribbon by coupling hexabromo substituted-propellane molecules on Au(111) and Ag(111).[4] In the structure, the C-Br bonds distant from the surface remained intact even after the reaction. The radical species were formed by tip-induced debromination and were also stabilized by either Br atom or fullerene molecule. For heteroatom substitution, we developed new on-surface reaction, which can synthesize graphene nanoribbon and covalent organic frameworks with silabenzene units by coupling Si atom and Br-substituted molecule (Figure1).[5] The heavier congeners of cyclic aromatic compounds have been studied as an elusive target product for organic synthesis due to their high reactivity at ambient temperature and difficult isolation. Thus, this result shows the advantage of on-surface synthesis.
References
[1] L. Grill, et al., Nat. Nanotechnol. 2, 687. (2007).
[2] S. Clair, D. G. de Oteyza, Chem. Rev. 119, 4717 (2019).
[3] L. Gross, F. Mohn, N. Moll, P. Lilkeroth, G. Meyer, Science 325, 1110 (2009)
[4] S. Kawai, et al Sci. Adv. 6, eaay8913 (2020).
[5] K. Sun et al, Nat. Chem. 15, 136 (2023).