Equipe Nanostructuration (NANO)
Responsable d'équipe-s :
Christian LOPPACHER VOIROL
Responsable-adjoint-e-s :
Lionel PATRONE
- Campus de Saint Jérôme, Marseille
Aile 1, niveau 5, Service 151
Salle des doctorants: 04.13.94.52.76
Salle IPE: 04.13.94.52.79
Salle AFM-STM: 04.13.94.52.45
- Campus ISEN Yncréa Méditerranée, Toulon
- The Nanostructuration Team is part of the inter-laboratory team Nano-Mat-Mol with CINaM laboratory.
- ISEN site in Toulon
Research Subjects:
- On-surface synthesis
- Electronic properties of organic/inorganic interfaces
- Molecules on wide-gap surfaces
- Surface Enhanced Raman spectroscopy and Near Field Optics
- Catalytic Scanning Probe Nanolithography (c-SPL)
- Press Releases:
-
- Research
On-surface synthesis is a newly developing field of research that aims at making use of well-defined solid surfaces as confinement templates to initiate chemical reactions. The concepts of supramolecular chemistry are here applied to provide well-defined functional surfaces from the “bottom-up” self-assembly of nanometer-sized elementary building-blocks. The interest for creating covalent nanoarchitectures directly on surfaces is manifold. On-surface synthesis gives access to original reactions mechanisms in mild conditions that would be not easily accessible in standard chemistry conditions. Also, it represents an efficient route to the formation of robust organic networks and 2D polymers. Finally, the full range of available surface science techniques can deliver exquisite characterization of the different reaction processes with atomic precision.
Selected publications:
Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis
S. Clair, D. de Oteyza, Chemical Reviews 119, 4717-4776 (2019)
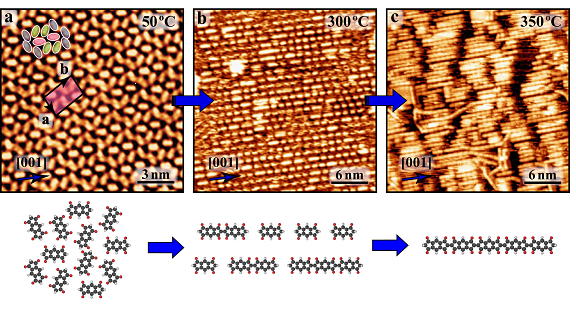
On-surface synthesis of aligned functional nanoribbons monitored by scanning tunneling microscopy and vibrational spectroscopy
N. Kalashnyk, K. Mouhat, J. Oh, J. Jung, Y. Xie, E. Salomon, T. Angot, F. Dumur, D. Gigmes, S. Clair, Nature Communications 8, 14735 (2017)
Growth of boronic acid based two-dimensional covalent networks on a metal surface in ultrahigh vacuum
S. Clair, M. Abel, L. Porte, Chemical Communications 50, 9627 (2014)
Electron spectroscopies like direct and inverse photoemission are powerful tools to explore the electronic properties of materials. One of the main aspects is their surface sensitivity making them particularly suited for the study of the interface between a thin organic film and an inorganic substrate. In the near ultra-violet range (UPS and IPES) the valence and conduction band are effectively probed. This can give valuable information about density of states of a thin film or the interface and on the adlayer/substrate energy level alignment. Such features are used in our group to study the interface bonding mechanisms and the charge injection barriers in organic/inorganic interfaces. Particularly, combining IPES and UPS the transport gap can be measured (see figure below, left panel).
XPS uses higher energy radiation and enables one to probe deeper electronic levels with elemental sensitivity. Moreover, since their binding energy depends on the valence charge distribution around an atom, energy shifts are used to probe the chemical environment of a given specie. Different oxidation states for –say- carbon within a given molecule can be detected by XPS, (see figure below, right panel) which can also shed light on the nature of the molecule-substrate interactions. Weaker interactions as intermolecular hydrogen bonds can also be detected.
Electron spectroscopies are performed in our group in two separate experimental setups: the IPES and the STM apparatus. In the first one, inverse (IPES) photoemission is available together with LEED, Auger, and standard preparation techniques. In the second UPS and XPS photoemission can be performed in parallel with in situ imaging by STM (LEED and preparation chambers also available). Moreover we regularly access to different synchrotron radiation facilities to perform high-resolution photoemission and photo-absorption experiments.
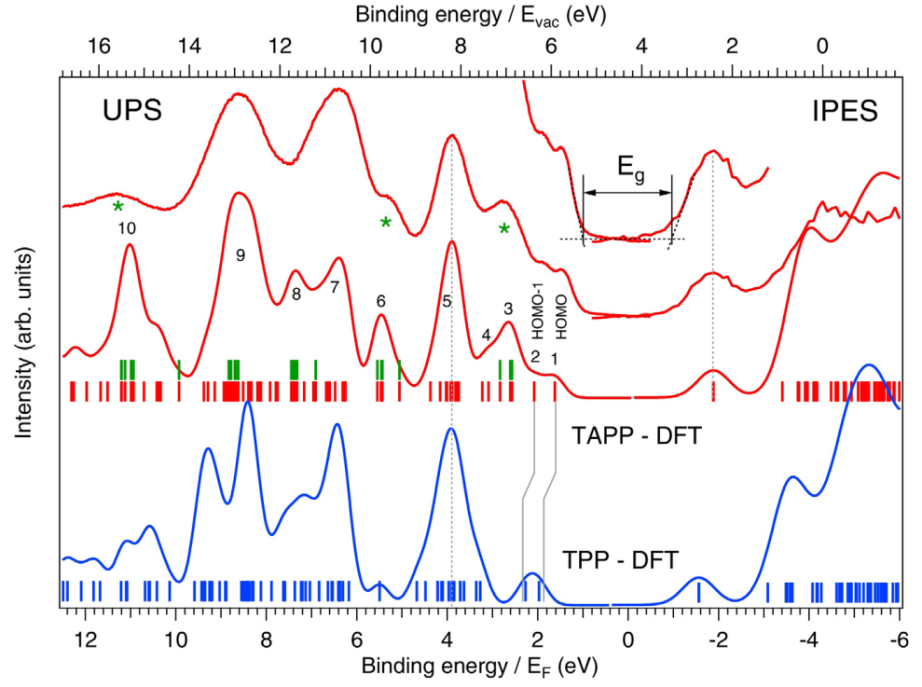
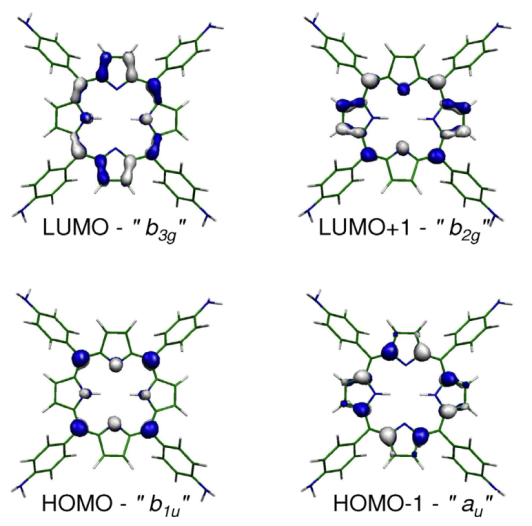
Direct and inverse photoemisison of the tetra(4-aminophenyl)porphyrin (upper spectrum). Comparison with DFT calculations (middle calculated spectrum) reveals that the presence of the two low-energy peaks in the filled states due to the lifting of the quasi-degeneracy of the HOMO and HOMO-1 in the non-substituted tetra-phenyl porphyrin (blue calculated spectrum). This is attributed to an increased HOMO orbital destabilization due to an enhanced electron-donor character of the phenyl substituents upon amino functionalization (see right panel). For more details see L. Giovanelli et al., J. Elec. Spectr. And Rel. Phen. 218, 40 (2017).
Probing the magnetic properties of 2D metal-organic systems by X-ray magnetic circular dichroism
π-conjugated macrocycles such as phthalocyanines and porphyrins hosting a single transition metal atom have shown great versatility in producing 2D magnetic arrays when adsorbed on well-ordered surfaces. This includes the possibility to modify the magnetic state of the metal atom through ferromagnetic or anti-ferromagnetic coupling to the substrate and by adsorption of smaller ligands (CO, O2). An alternative approach for the synthesis of magneto-organic nanostructures consists in manipulating the magnetic properties of transition metal atoms through selective bonding to functional ligands in surface-supported, self-assembled metal-organic networks. Thanks to the availability of synchrotron light sources and because of its capability of sensing the magnetic properties of a system in a element-selective way and down to the impurity level, X-ray magnetic circular dichroism (XMCD) is a preferred tool to study organic magnetic molecules. Through a close collaboration with the Magnetism (A. Savoyant) and Theory (R. Hayn) groups of IM2NP, we aim at investigating the magnetic properties of surface-supported 2D metal-containing organic systems using XMCD. In a first work we have revealed a ferromagnetic coupling between magnetic centers embedded in a metal-organic network on two different metal substrates. Angle and magnetic-field dependent measurements allowed to extract application-relevant parameters such as the magnetic coupling and single ion anisotropy.
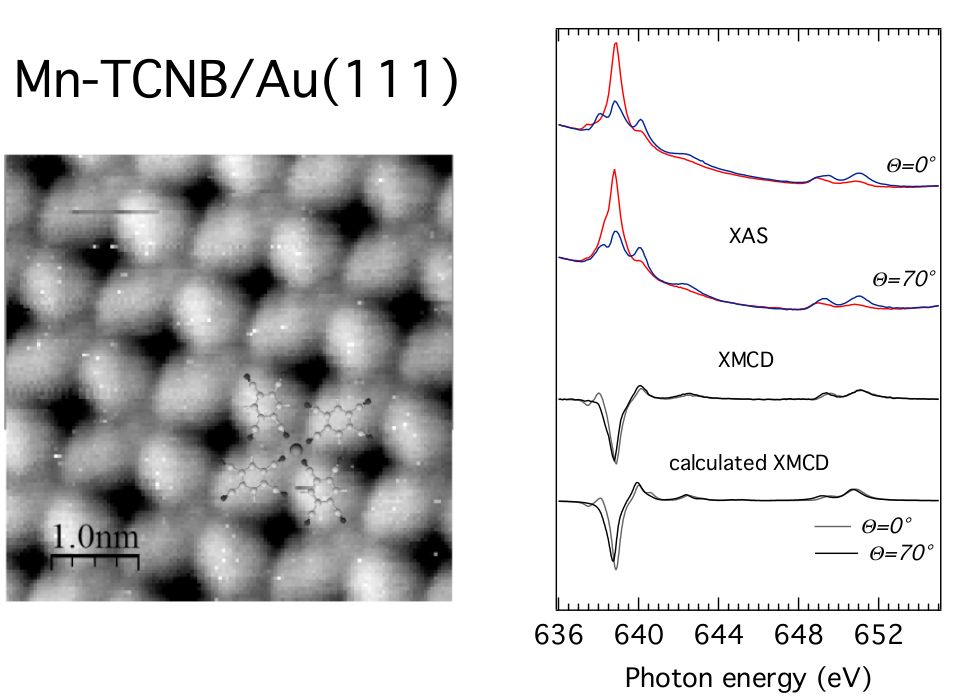
STM image of the Mn-TCNB metal-organic network on Au(111) and XAS spectra at different incident angle taken with opposite alignment between the circular polarization vector and applied magnetic field (6 T). The resulting XMCD spectra are modeled by multiplet calculations.
Molecules on semiconductors
Silicon Carbide (SiC) is a promising material for high-voltage, high-temperature and high-frequency electronic devices because of its wide bad-gap (~3eV), extreme hardness and thermal stability. Moreover, the diversity of its surface reconstructions triggers high interests in both the fundamental understanding of its surface electronic structure and possible applications. The interaction of organic molecules with SiC surfaces is of particular interest since it may lead to e.g. biosensors or optoelectronic devices. Besides, studying the electronic structure of organic molecules adsorbed on reconstructed semi-conducting surfaces may improve our understanding of organic thin film growth and molecular self-assembly mechanisms on semiconductor surfaces. In a recent study we have pursued a long-standing issue such as the binding of the ubiquitous C60 molecule on a Si-terminated surface, namely the 3x3-SiC(0001). High resolution synchrotron radiation XPS and UPS revealed that C60 is chemisorbed by orbital hybridization between the highest-occupied molecular orbital (HOMO) and the pz orbital of Si adatom at the apex of the tetramers.
Left: experimental and simulations of sub-ML C60/3x3-SiC(0001). The abundance of energetically-closed adsorption sites (different colored disks representing C60 molecules) none of which holds a convenient conmensurability explains the lack of long-range order. Right: the UPS spectrum displays a high binding energy component suggesting an orbital hybridization to ensure the molecule-substrate bonding.
Selected publications:
Peculiar covalent bonding of C60/6H-SiC(0001)-(3×3) probed by photoelectron spectroscopy
F.C. Bocquet, L. Giovanelli, Y. Ksari, T. Ovramenko, A. J. Mayne, G. Dujardin, F. Spillebout, P. Sonnet, F. Bondino, E. Magnano and J.-M. Themlin, J. Phys.: Condens. Matter 30, 505002 (2018)
L. Giovanelli, H.-L. Lee, C. Lacaze-Dufaure, M. Koudia, S. Clair, Y.-P. Lin, Y. Ksari, J.-M. Themlin, M. Abel, A.A. Cafolla, J. Elec. Spectr. And Rel. Phen. 218, 40 (2017)
Nitrogen-doping processes of graphene by a versatile plasma-based method
Y.-P. Lin, Y. Ksari, J. Prakash, L. Giovanelli, J.-C. Valmalette, J.-M. Themlin, Carbon 73, 216 (2014)
Nitrogen-doping processes of graphene by a versatile plasma-based method
Y.-P. Lin, Y. Ksari, J. Prakash, L. Giovanelli, J.-C. Valmalette, J.-M. Themlin, Carbon 73, 216 (2014)
Since 2009, the experimental part of our research activities are focused on the structural, electronic and optical properties of organic molecules adsorbed on bulk dielectrics (ionic crystals) surfaces in the mono-layer regime. These are gathered into three topics:
- Structure and growth of supramolecular assemblies
- Optical properties of adsorbed organic layers by Differential Reflectance Spectroscopy
- On-surface polymerization processes
Our experimental tools are non-contact Atomic Force Microscopy (nc-AFM), Kelvin Probe Force Microscopy (KPFM), as well as Differential Reflectance Spectroscopy (DRS) in ultra-high vacuum (UHV).
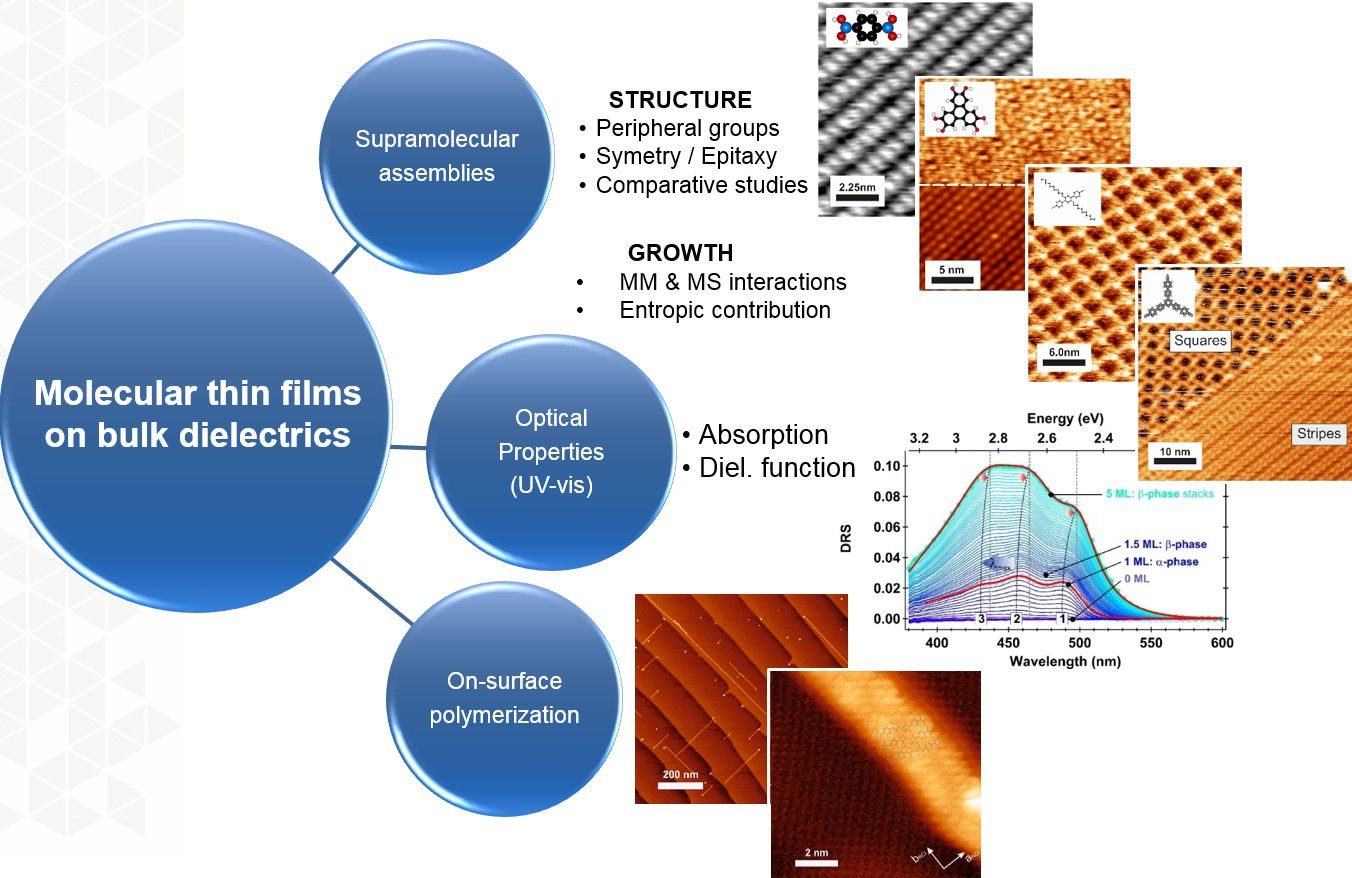
Figure : Overview of our experimental research activities
I.1- Structure and growth of supramolecular assemblies:
Our expertise is focused on the study of the structural properties of adsorbed organic molecules grown in the mono-layer regime at the surface of ionic crystals, such as alkali halides (NaCl, KCl, RbCl, KBr). At the surface, the molecules interact with each other without forming covalent bonds, but specific, long-range (~ a few angströms), hence weaker, intermolecular interactions forming so-called supramolecular assemblies.
The influence of:
- the molecule shape and symmetry
- the molecule’s peripheral groups
- the relative behavior upon adsorption of a given tecton on different substrates
- the molecule-molecule and molecule-substrate interactions
was investigated on a large set of molecules [1-4]. Our experimental results are systematically compared to Density Functional Theory-based calculations as-performed by colleagues from national or international institutions, which brings further insights from the understanding of the structures.
Thus, original types of growths were found out (line on line epitaxy). Polar peripheral groups and molecular entropy were also found to be key mechanisms to steer the ordered growth of the supramolecular assemblies.
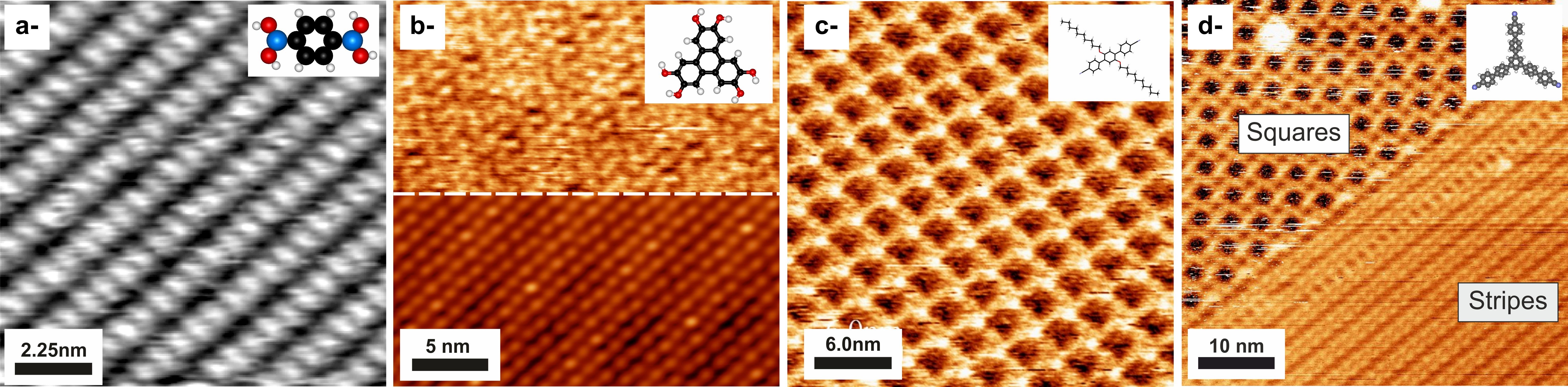
Figure : Examples of organic supramolecular assemblies on alkali halides substrates, as measured by nc-AFM. a- Boronic acid on KCl(001), after [1]. b- Hexa-hydroxy triphenylene on KCl(001), after [2]. c- 1,4-bis(cyanophenyl)-2,5-bis(decyloxy) benzene molecule on KCl(001), after [3]. d- 1,3,5-tri-(4-cyano-4,4-biphenyl) benzene molecule on KCl(001), after [4].
References :
[1] R. Pawlak et al., J. Phys. Chem. C 114, 9290 (2010)
[2] F. Bocquet et al., Phys. Rev. Lett. 108, 206103 (2012)
[3] A. Amrous et al., Adv. Mater. Interf., 1400414 (2014)
[4] J. Gaberle et al., J. Phys. Chem. C 121, 4393 (2017)
I.2- Optical properties of adsorbed organic layers by DRS:
Differential Reflectance Spectroscopy (DRS) is a real-time, UV-visible, spectroscopic method allowing for the measure of the reflectance changes of a surface upon adsorption of additional material. A DRS setup is adapted on our UHV setup (see "instrumental developments" section below). Hence, the DR spectra are recorded on materials grown in situ.
DRS is used to investigate the optical response of the organic adlayers adsorbed on insulating substrates such as alkali halide single crystals. The reflected specular light intensity is measured as a function of the wavelength, then normalized to the one of the bare substrate, which ultimately gives access to the absorption properties of the adsorbed adlayer.
The as-measured DR spectra are interpreted as functions of:
- the intrinsic molecular absorption (molar extinction coefficient)
- the geometric parameters of the deposit on the substrate (molecular unit cell, thickness, roughness, number of layers…)
The intrinsic molecular absorption are derived from regular spectroscopic methods (absorption / fluorescence) of the molecular tectons in solvants. The geometric parameters are derived from non-contact AFM experiments performed in the same UHV chamber. A typical set of DR spectra is shown in the fig. below, after [1].
An important piece of effort for the modelization of the DR spectra has been carried out that allows us to extract the dielectric function of each organic layer.
Further details on the experimental and theoretical aspects of our approach to DRS can be found here and here, respectively.
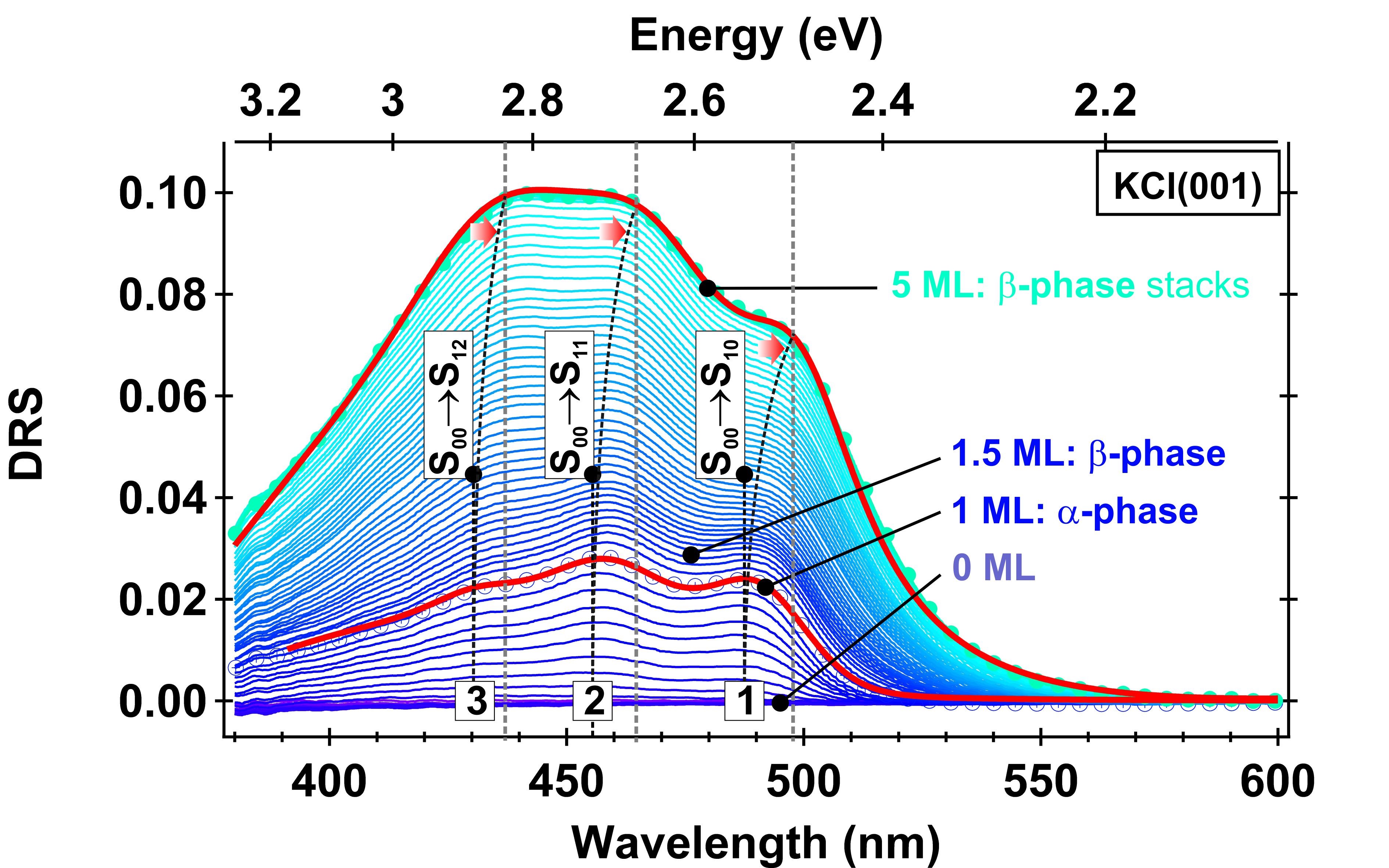
Figure : Reflected specular intensity (DRS signal) as a function of the wavelength measured during the growth of molecular layers of bis-pyrene molecules from 0 to 5ML (~ 10 nm) adsorbed on a KCl(001) substrate. Non-contact AFM structural data tell us the molecules form 2 phases (a-phase up to 1ML, b-phase above). Vibronic replicas are visible, which change as a function of the growth (1,2,3). Good-quality fitting (red curves) can only be achieved if a mix of a and b phases is considered (see [1]).
References :
[1] F.Bocquet et al. Phys. Rev. B 97, 235434, (2018).
I.3- On-surface polymerization processes:
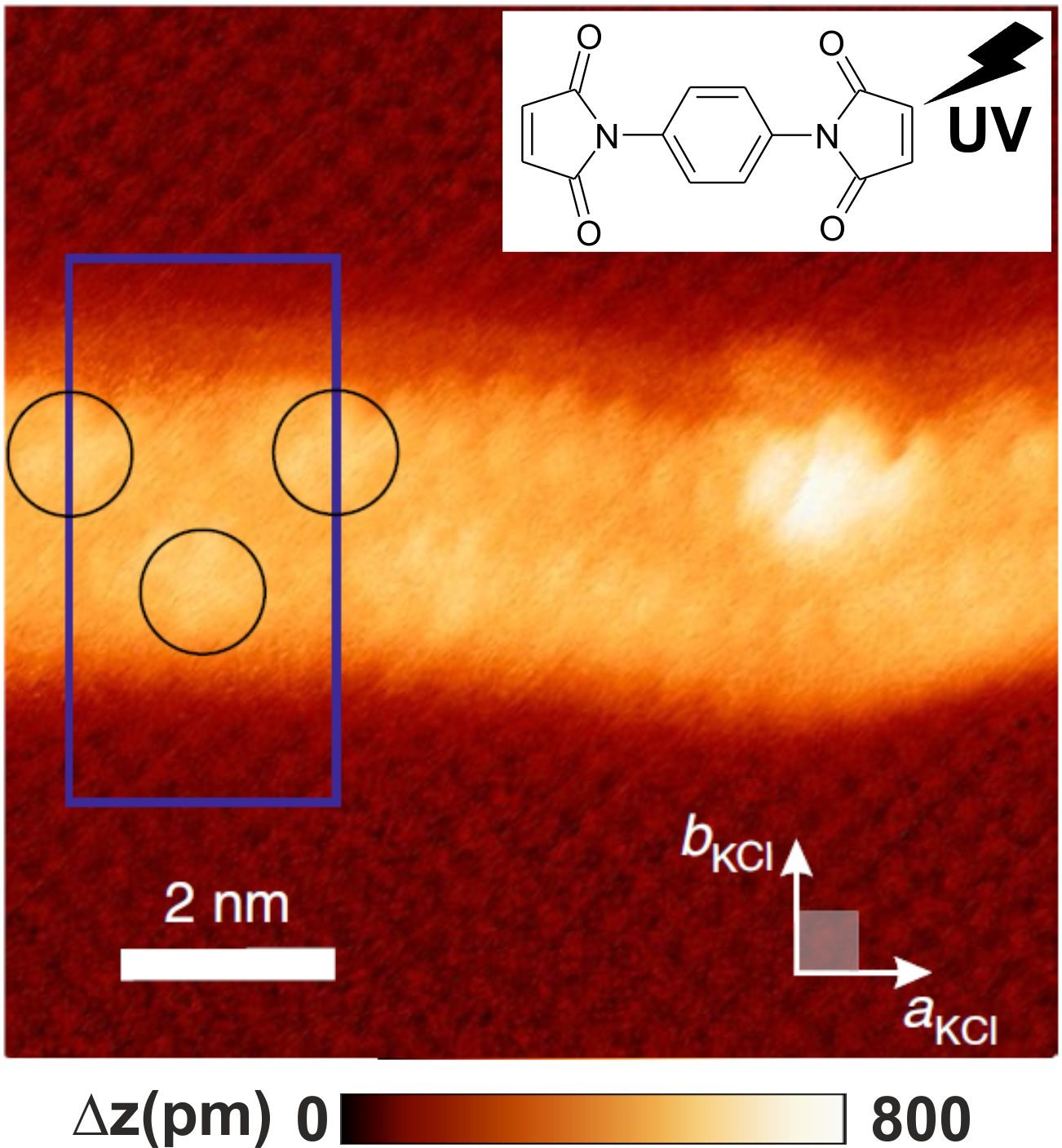
Figure : Dimaleimides molecules polymerized on a KCl(001) surface after UV irradiation. The nc-AFM image allows us to resolve the substrate down to the atomic scale as well as the polymer fiber with molecular resolution (after [1]).
References :
[1] F.Para et al. Nature Chemistry 10, pages1112–1117 (2018). DOI: 10.1038/s41557-018-0120-x
_________________________________________________________________________________
Our UHV setup is operational since December 2008. It consists of two inter-connected UHV chambers (base pressure 10-10 mbar), the “preparation” and the “analysis” chamber (P- and A-chamber, respectively).
The P chamber includes:
- two built-in molecular evaporators facing quartz micro-balances
- a heating stage allowing for the electron bombardment (up to 1500°C)
- an additional heating stage for temperatures up to 600°C
- a cleavage stage for cleaving in-situ the ionic crystals
- an ion gun
- the DRS setup (see "instrumental developments" section below).
The A-chamber includes:
- the VT-AFM from Omicron®. The instrument was optimized in several manners (see “instrumental developments” section below).
- a low current LEED-Auger Electrons spectroscopy setup from OCI MicroEngineering®
The driving electronics of the VT-AFM is a R9 from RHK®. It includes the PLLPro Control System for non-contact AFM and KPFM operations, a Piezo Motor Control unit for driving the macroscopic displacements of the sample, and the unit for driving the piezos. The interfacing between the VT-AFM and RHK's electronic was discussed in [1].
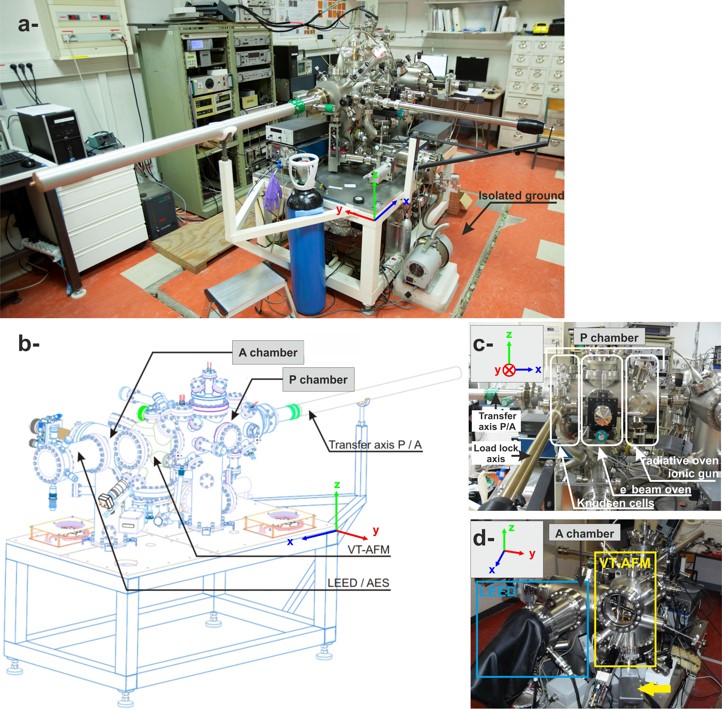
Figure : The UHV setup.
References :
[1] L.Nony et al. http://www.rhk-tech.com/wp-content/uploads/2016/02/R9_Omicron_ApplicationNote.pdf, RHK Technology Application Note (2015).
II.2- Instrumental developments:
II.2.a- Optimizations of the UHV VT-AFM:
The VT-AFM is a beam deflection-based instrument (probes=cantilevers) that was optimized to improve its nc-AFM/KPFM imaging performances. The AFM includes a two-stages preamplifier, the first stage being in UHV, beneath the 4-quadrants Photo Sensitive Detector (PSD). The second stage is located ex-situ and used for the conditioning of the I/V-converted PSD signals. It is referred to as the Mathbox and computes the vertical and lateral deflections of the cantilever.
A poster summarizing these optimizations can be found here.
The list of specifications of the instrument when used at room temperature is detailed hereafter:
- Light: Superlum diode @ 844nm (coherence length ~47nm); fiber output power: 4mW
- In-situ preamplifier (first stage): BDW = 3 MHz, I/V gain = 2200. Electrical consumption: 50 mW (per PSD channel). PSD reverse bias = -15V
- Mathbox (second stage): Calculation of the vertical and lateral deflection of the cantilever. Differential gain: x1.. x20. BDW = 1..5 MHz
- Noise floor on the cantilever oscillation signal: The noise floor of the instrument is 150 fm/ÖHz. Integration on the detection bandwidth (3 MHz) yields a rms noise on the oscillation amplitude of 300 pm.
- Oscillation amplitude: At 150 kHz (typical resonance frequencies of the cantilevers that are used), 1 nm oscillation amplitudes (peak-to-peak) provide enough stability for nc-AFM operation.
- Mechanical noise floor in the room: The AFM is located on a ground that is isolated by a trench from the rest of the building. The mechanical noise floor is 1 nm.s-2 for frequencies up to 10 Hz.
- Vertical noise (z channel, topography): The vertical noise is 5 pm typ. (see figure below).
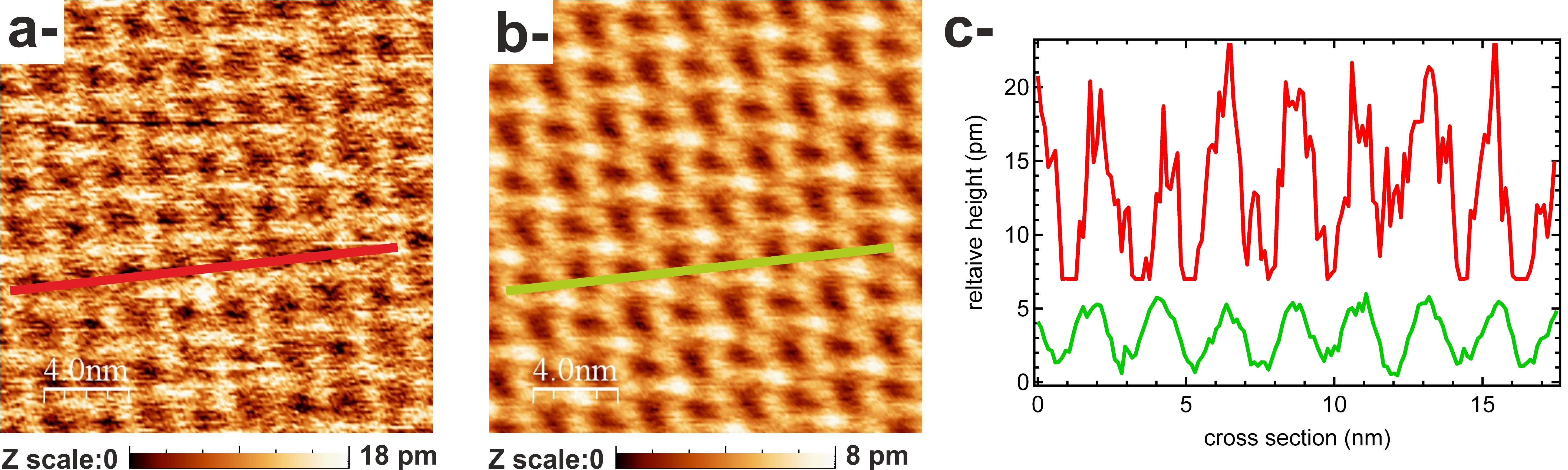
Figure : nc-AFM topographical images of 1,4-bis(cyanophenyl)-2,5-bis(decyloxy) benzene molecules on KCl(001). a- Raw data. b- Filtered data. c- Cross sections along red and green lines in a- and b- respectively showing the Z level of noise.
II.2.b- Differential reflectance spectroscopy:
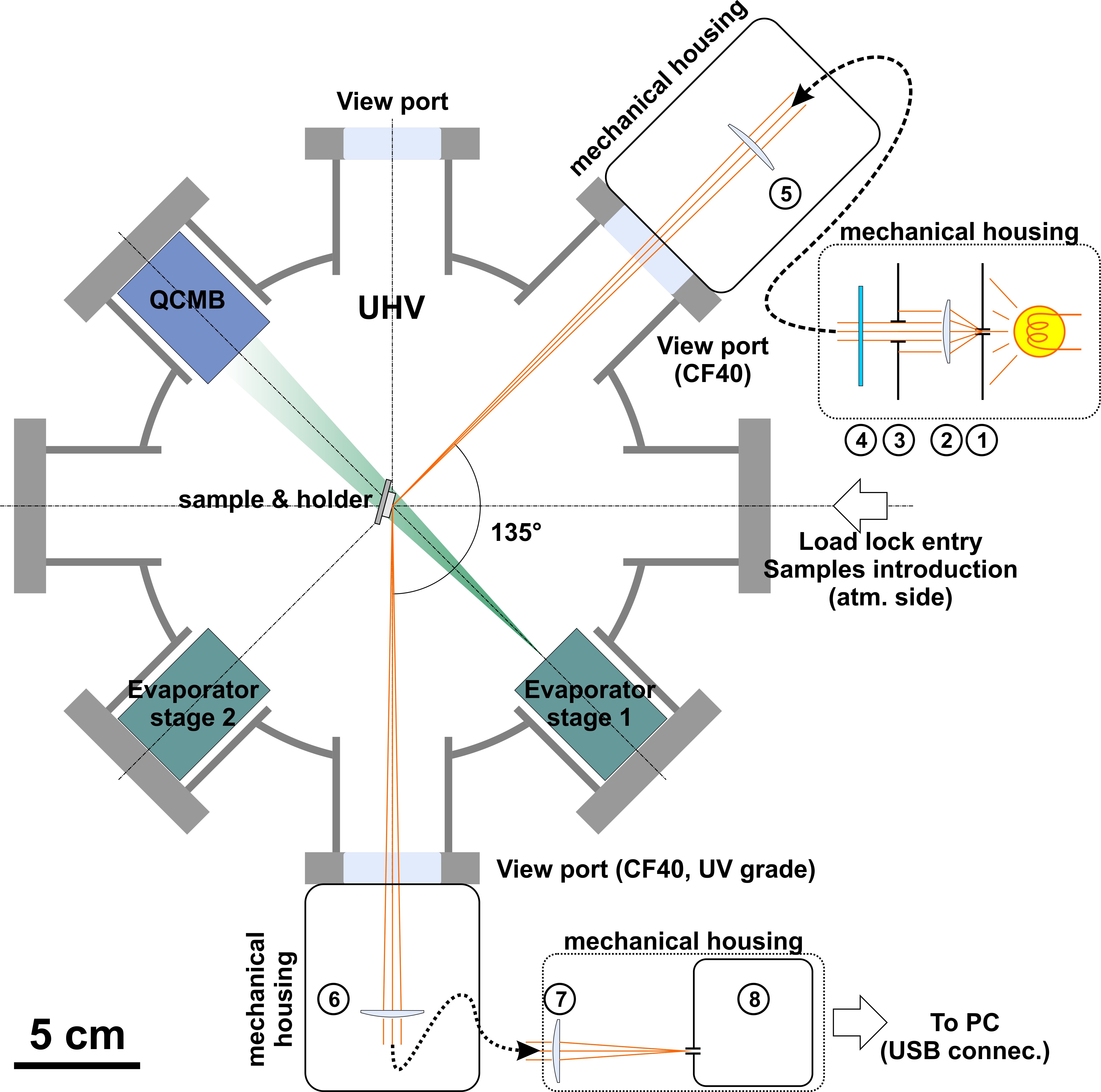
Figure: Implementation of the DRS setup on the P-chamber.
References :
[1] F.Bocquet et al. Phys. Rev. B 97, 235434, (2018).
II.2.c- Developments with quartz tuning forks-based nc-AFM probes:
Quartz tuning forks (QTF-) are commonly used as probes for combined nc-AFM/STM applications at low temperature. Nonetheless, they still remain controversial probes, as they can beget important interplay between frequency shift, damping and AC tunneling current (due to the oscillation of the tip) signals. The reasons for such a cross-talk remain under debate.
We have proposed an interpretation framework for that cross-talk [1]. It is inferred that AC tunneling current radiates an electromagnetic fields that penetrates the QTF's prong end and gets coupled to the piezoelectric tensor of the material. This unexpected field yields an additional deformation of the prong's end which is converted into motion, hence in additional, unexpected, oscillation of the QTF. This ultimately fakes both the information on the tip-surface distance, hence the frequency shift, but also the damping.
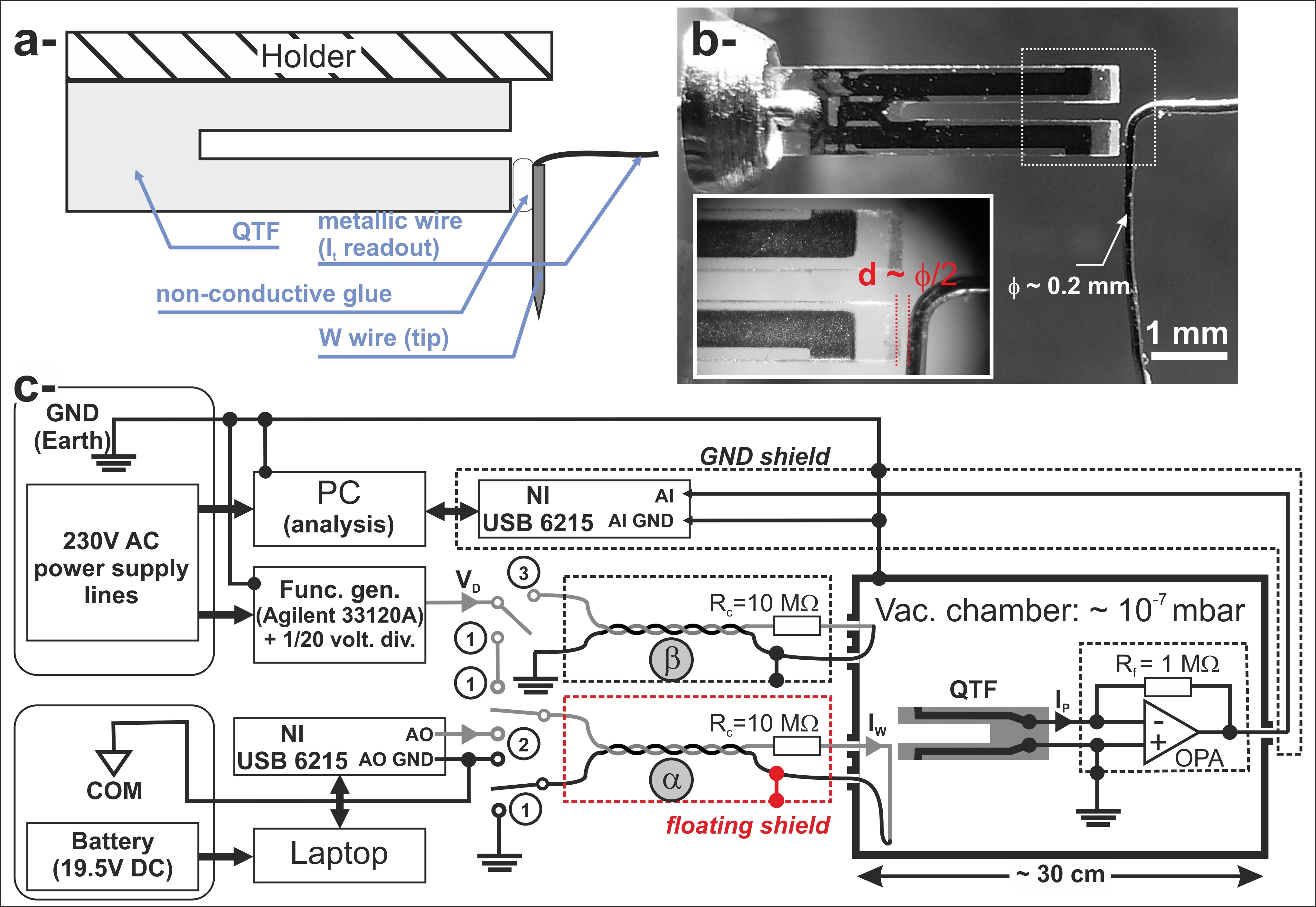
Figure: Scheme of the instrumental development on QTF
References :
[1] L.Nony et al. Phys. Rev. B 94, 115421 (2016).
_________________________________________________________________________________
III. Modelization:
Along with our experimental activities, an important part of our research was also focused on modelization and numerical developments in relation with the systems that were investigated experimentally.
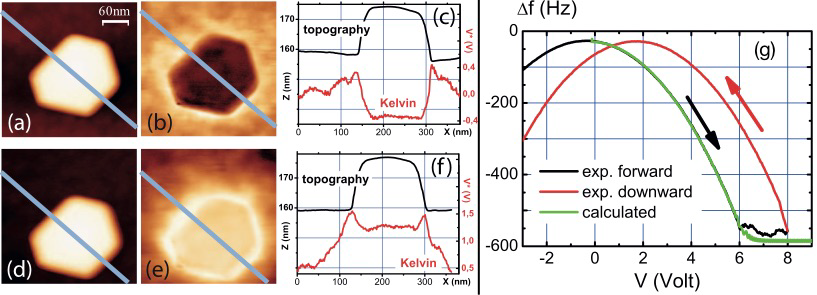
We have developed an analytical approach to the LCPD with the goal in mind to better understand the atomic-scale contrast observed while Kelvin Probe Force Microscopy measurements (KPFM) on KBr(001) [1]. The approach relies on the determination of the electrostatic force occurring between a metallic tip carrying a small metallic cluster in topmost position and the bulk ionic crystal below. The approach states that the ions at the surface undergo dynamic displacements (ionic polarization) under the influence of the inherent modulation of the bias voltage between the tip and the crystal that is required by the KPFM technique. The force, with a short-range nature, is site-dependent and has the lateral periodicity of the Madelung surface potential, i.e. the one of the underlying ionic lattice. It does not only scale quadratically with the applied bias voltage, but also linearly. The analytical approach to the KPFM method, i.e. actually the analytical expression of the DC potential that nullifies this force, namely the LCPD, shows that the latter force is responsible for the atomic-scale contrast in KPFM but that any quantitative connection to the physical observables such as the Madelung surface potential, or the local work function can be made owing to the convolution of the detected LCPD by the tip’s geometrical parameters [1,2].
References :
[1] F.Bocquet et al., Phys.Rev.B 24, 1791 (2008)
[2] L.Nony et al. Nanotechnology 20, 264014 (2009).
III.2- Simulating the nc-AFM/KPFM setup.
Following the analytical developments, we have implemented the KPFM operational mode within the core of an accurate numerical implementation of the nc-AFM setup, the so-called nc-AFM simulator [1]. With the fully numerical nc-AFM/KPFM setup, it is possible to simulate spectroscopic curves as well as topographical and LCPD images. In collaboration with Prof. Adam Foster from Tampere University (Finland), we have used the nc-AFM/KPFM simulator in combination with atomistic force fields computed between a tip with a realistic geometry and the NaCl(001) surface. This large computational effort strengthen most of the analytical results and led to the conclusion that the simultaneous occurrence of the atomic-scale topographical and KPFM contrast is possible (no experimental artifact, no cross coupling between channels), but that the latter relies on the dynamic polarization of the ions at the tip-surface interface. This makes any quantitative measurement of the Madelung surface potential possible [2,3]. However, the “chemical” periodicity remains preserved. Further details can be found here.
References :
[1] L.Nony et al., Phys.Rev.B 74, 235439 (2006)
[2] L.Nony et al., Phys.Rev.Lett. 103, 036802 (2009).
[3] L.Nony et al. Chapter in the book «Kelvin Probe Force Microscopy», (Eds. Sascha Sadewasser & Thilo Glatzel), Springer-Verlag Berlin Heidelberg (2011), DOI 10.1007/978-3-642-22566-6
III.3- Methodology for fitting the DR spectra
References :
[1] F.Bocquet et al. Phys. Rev. B 97, 235434, (2018).
Raman spectroscopy is a vibrational spectroscopy based on inelastic light scattering. As such, it is a powerful analysis tool in the field of materials. Nevertheless classical Raman spectroscopy is limited by its very low cross section. Since 10 years, our team is developing a research activity based on Surface Enhanced Raman Spectroscopy (SERS). This technique is based on plasmonic nano-antenna, usually gold nanoparticles. The Raman signal is strongly enhanced by those antennas, increasing the final cross section of the Raman process. It is thus possible to detect molecules in very low concentration. An example of such nano-antenna can be seen in the figure below.
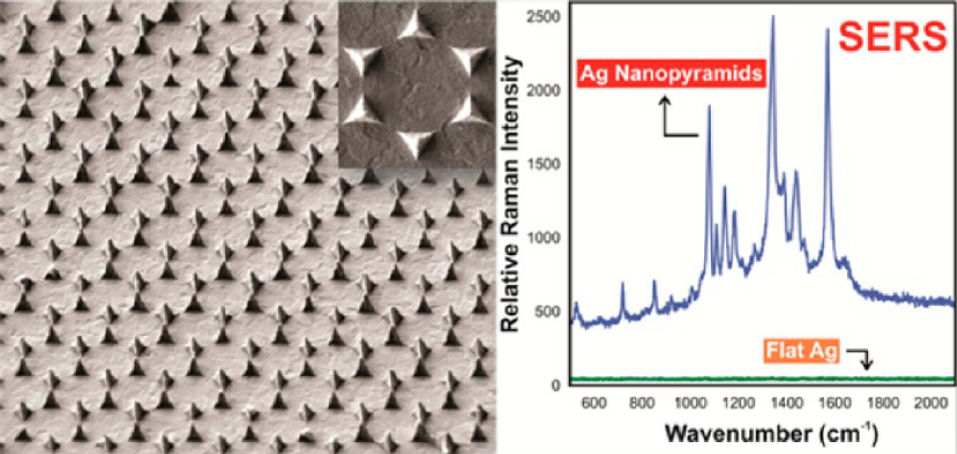
Figure: An exemple of 3D plamsonic nano-antennas for SERS. This work has been performed in collaboration with the University of WESTERN Ontario
In addition to SERS, our team is developing an apertureless scanning optical microscope based on a single nano-antenna fixed at the APEX of a scanning probe. This approach is named Tip Enhanced Raman Spectroscopy (TERS). It has two major advantages: a high sensitivity associated to a high spatial resolution (typically a few nanometers).
All those works are performed in collaboration with several laboratories (UWO and INRS, Canada and PIIM, France).
Selected publications:
J. Plathier, A. Merlen, A. Pignolet, A. Ruediger, J Raman Spectrosc. 2017; 48 (12), 1863-1870.
A. Merlen, C. Pardanaud, S. Coussan, C. Panagiotopoulos, O. Grauby, Carmen M. Ruiz, J Raman Spectrosc. 2018 ;49 (7), 1184-1189.
In catalytic scanning probe lithography (cSPL), the tip of an AFM is modified to accommodate a catalyst, usually a metal deposited as a thin film or in nanoparticule form. Part of the reactants is provided on a surface in the form of a self-assembled monolayer (SAM) bearing reactive endgroups, and the remaining reactants are solvated in the surrounding liquid. A chemical coupling reaction is initiated locally by mechanical application of the catalytic tip and various chemical compounds can be grafted selectively, with a spatial resolution in the order of a few tens of nm. The technique can be extended to the use of homogeneous catalyst immobilized onto the tip upon modification with an appropriate ligand. The use of a homogeneous catalyst opens in principle the way to a large variety of chemical reactions. It was shown that the catalytic activity of the tip-supported catalyst was barely affected during the reaction process, and large areas could be covalently modified without significant loss of the reactivity.
This thematic is developed in collaboration with Jean-Luc Parrain and Olivier Chuzel from iSm2 laboratory.
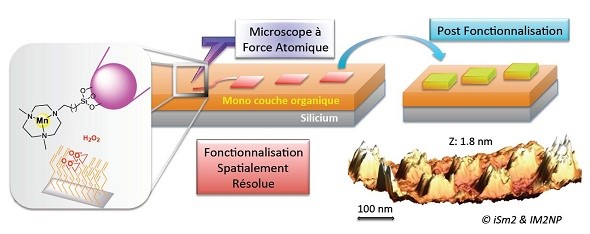
Selected publications:
J. Botton, K. Gratzer, C. François, V. Mesquita, L. Patrone, T.S. Balaban, S. Clair, J.-L. Parrain, O. Chuzel, Chemical Science 9, 4280 (2018)
V. Mesquita, J. Botton, D.A. Valyaev, C. François, L. Patrone, T.S. Balaban, M. Abel, J.-L. Parrain, O. Chuzel, S. Clair, Langmuir 32, 4034 (2016)
D.A. Valyaev, S. Clair, L. Patrone, M. Abel, L. Porte, O. Chuzel, J.-L. Parrain, Chemical Science 4, 2815 (2013)
|
Date |
Project name |
Collaborators |
|
2024-2028 ANR PRC |
PESOS: Imaging the Photo-Excited Electronic States at the Molecular Scale |
Institut Néel B. Grévin) |
|
2023-2025 AMIDEX |
ATOPRINT: Atomically-printed 3-dimensional molecular assembly |
ICR (D. Gigmes) |
|
2022-2026 ANR PRC |
Light4Net: Photo-Induced On-Surface Synthesis to Elaborate Highly-Ordered Covalent Structures on Insulating Substrates |
FEMTO-ST (F. Cherioux) |
|
2022-2026 ANR PRC |
GANESH: Graphene Nanomesh |
CEA-IRAMIS (S. Campidelli) LUMIN-ENS (JS. Lauret) CEA-SPEC (S. Latil) |
|
2021-2025 ANR PRC |
CROSS: Controlled Reactivity of Sulfoxides on Insulating Surfaces |
CEMES (C. Kammerer) LHFA (D. Madec) |
|
2017-2022 ANR PRC |
DUALITY: Surface-directed Elaboration of Two-dimensional Nanoporous Covalent Organic Frameworks |
ICR (F. Dumur) PIIM (E. Salomon) |
|
2018-2020 AMIDEX |
MATMOL: Acquisition d’un système combiné AFM/STM ultra-vide basse température pour l’étude de MATériaux MOLéculaires nanostructurés avec résolution intramoléculaire |
ICR (D. Gigmes) |
|
2017-2020 Région PACA |
MOLOS: Acquisition of a Low-Temperature AFM/STM for the study of one- and two-dimensional organic systems with sub-nanometer resolution. (Acronym MOLOS, MOLecules On Surfaces) |
ICR (D. Gigmes) |
|
2016-2020 ANR PRCI |
PHOTONET: On-surface synthesis of covalent networks with integrated optical functions |
ISM2 (JL. Parrain) RIKEN (Y. Kim) |
|
2013-2016 AMIDEX |
REBICOF: Two-dimensional functionalized covalent organic frameworks |
ICR (D. Gigmes) |
|
2013-2016 ANR PRC |
CASPARES: CAtalysis SPAtially REsolved on a Surface |
ISM2 (JL. Parrain) |
|
2010-2013 ANR JCJC |
COVANET: Functionalized covalent 2D networks for immobilization of transition metal atoms |
ICR (D. Gigmes) |
Mots clefs
Surfaces and interfaces
Scanning Probe Microscopy
Photoelectron Spectroscopy
Raman Spectroscopy
Scanning Probe Microscopy
Photoelectron Spectroscopy
Raman Spectroscopy
Collaborations
 The Nanostructuration Team is part of the inter-laboratory team Nano-Mat-Mol with CINaM laboratory.
The Nanostructuration Team is part of the inter-laboratory team Nano-Mat-Mol with CINaM laboratory.




![]()
Actualités
Séminaire 23/09/19 - David Martrou - Controlling the electric charge of gold nanoplatelets on an insulator by field emission nc-AFM
Salle des séminaires de l'Im2np, campus de Saint-Jérôme, aile 1, niveau 6 service 161
-

Séminaire Maria Dekermenjian - 16 Mai 2019 - Effet de la spectroscopie Raman exaltée par effet de pointe (TERS) sur les modes Raman du premier ordre
Amphi Y1-012, campus de la Garde, Université de Toulon
-
Séminaire 03/04/19 - Sébastien Gauthier - CEMES, Toulouse
Salle des séminaires de l'Im2np, campus de Saint-Jérôme, aile 1, niveau 6 service 161
-

Séminaire Nataliya Kalashnyk - 26 Février 2019 - GeePs, Gif sur Yvette
Salle des séminaires de l'Im2np, campus de Saint-Jérôme, aile 1, niveau 6 service 161
-

Séminaire Azza Hadj Youssef - 14 Février 2019 - Manifestation de l'effet flexoéléctrique dans les couches minces de titanate de strontium.
Salle Y1-012, Campus de la Garde, Université de Toulon
-
Séminaire Hocine Khemliche - 5 décembre 2018- Modes d’organisation d’interfaces organique/inorganique révélés en temps réel par diffraction d’atomes rapides en incidence rasante.
Salle des séminaires de l'Im2np, campus de Saint-Jérôme, aile 1, niveau 6 service 161
-
Franck BOCQUET
permanent
Sylvain CLAIR
Directeur de recherche CNRS
Virginie GADENNE
Non permanent
Luca GIOVANELLI
permanent
Younal KSARI
Maître de conférences
Christian LOPPACHER VOIROL
permanent
Jean-Marc THEMLIN
permanent
Héla MREZGUIA
Non permanent
Laurent NONY
Maître de conférences
Franck PARA
Ingénieur de recherche - PhD
Lionel PATRONE
permanent
Publications
| Publications |
|---|
| Andrés Lombana, Songpol Chaunchaiyakul, Olivier Chuzel, Denis Hagebaum-Reignier, Jean-Luc Parrain, Franck Bocquet, Laurent Nony, Christian Loppacher, Federica Bondino, Elena Magnano, Hiroshi Imada, Emiko Kazuma, Yousoo Kim, Luca Giovanelli, Sylvain Clair, Competing pathways to aromaticity governed by amine dehydrogenation and metal–organic complexation in on-surface synthesis, Chemical Science, 2025, 16, pp.3198 - 3210 (10.1039/d4sc07550a) (hal-04944948) |
| Laurent Nony, Sylvain Clair, Daniel Uehli, Aitziber Herrero, Jean-Marc Themlin, Andrea Campos, Franck Para, Alessandro Pioda, Christian Loppacher, Stiffness calibration of qPlus sensors at low temperature through thermal noise measurements, Beilstein Journal of Nanotechnology, 2024, 15, pp.580 - 602 (10.3762/bjnano.15.50) (hal-04729711) |
| Elie Geagea, Daniel Medina-Lopez, Luca Giovanelli, Laurent Nony, Christian Loppacher, Stéphane Campidelli, Sylvain Clair, Growth Mechanism of Chevron Graphene Nanoribbons on (111)-Oriented Coinage Metal Surfaces, Journal of Physical Chemistry C, 2024, 128, pp.8601-8610 (10.1021/acs.jpcc.4c01080) (hal-04729690) |
| Yingzheng Gao, Dimitris Mousadakos, Tommaso Gorni, Luca de' Medici, Sylvain Clair, Dimitri Roditchev, Stéphane Pons, Manipulation of the Magnetic State of a Porphyrin-Based Molecule on Gold: From Kondo to Quantum Nanomagnet via the Charge Fluctuation Regime, ACS Nano, 2023, 17, pp.9082-9089 (10.1021/acsnano.2c12223) (hal-04234398) |
| D. Nikolaievskyi, M. Torregrosa, Alexandre Merlen, Sylvain Clair, Olivier Chuzel, Jean-Luc Parrain, T. Neisus, M. Cabie, C. Martin, C Pardanaud, Wrinkling and crumpling in twisted few and multilayer CVD graphene: High density of edge modes influencing Raman spectra, Carbon, 2023, 203, pp.650-660 (10.1016/j.carbon.2022.12.010) (hal-03887634) |
| Yingzheng Gao, Sergio Vlaic, Tommaso Gorni, Luca de' Medici, Sylvain Clair, Dimitri Roditchev, Stéphane Pons, Manipulation of the magnetic state of a porphyrin-based molecule on gold: From Kondo to quantum nanomagnet via the charge fluctuation regime, ACS Nano, 2023, 17, pp.9082-9089 (10.1021/acsnano.2c12223) (hal-04040024) |
| Nataliya Kalashnyk, Adam Hassan Denawi, Frédéric Dumur, Didier Gigmes, Xavier Bouju, Sylvain Clair, Self-assembly of s-indacene-tetrone on Cu(111): molecular trapping and patterning of Cu adatoms, Physical Chemistry Chemical Physics, 2023, 25, pp.10591–10598 (10.1039/D3CP00358B) (hal-04065918) |
| Fatima Hussein, Corentin Pigot, Francisco Romero Lairado, Marco Minissale, Eric Salomon, Thierry Angot, Frédéric Dumur, Malek Nechab, Didier Gigmes, Sylvain Clair, Luca Giovanelli, On-surface homocoupling reactivity of a chiral bifunctional bromoindanone molecule on Cu(111), New Journal of Chemistry, 2022, 46, pp.22869-22876 (10.1039/D2NJ04708J) (hal-03888491) |
| Luca Giovanelli, Rémy Pawlak, Fatima Hussein, Oliver Maclean, Federico Rosei, Wentao Song, Corentin Pigot, Frédéric Dumur, Didier Gigmes, Younal Ksari, Federica Bondino, Elena Magnano, Ernst Meyer, Sylvain Clair, On-surface synthesis of unsaturated hydrocarbon chains through C-S activation, Chemistry - A European Journal, 2022 (10.1002/chem.202200809) (hal-03712529) |
| Nataliya Kalashnyk, Sylvain Clair, Self-accommodating honeycomb networks from supramolecular selfassembly of s-indacene-tetrone on silver surfaces, Langmuir, 2022, 38, pp.1067-1071 (10.1021/acs.langmuir.1c02640) (hal-03585485) |
| Thomas Leoni, Laurent Nony, Elena Zaborova, Sylvain Clair, Frédéric Fagès, Franck Para, Alain Ranguis, Conrad Becker, Christian Loppacher, Stereoisomeric selection upon adsorption: A structural and optical study of curcuminoid derivatives on ultrathin films of KCl on Au(111) and on KCl(001) bulk, Physical Review B, 2021, 104 (10.1103/PhysRevB.104.205415) (hal-03424085) |
| Alain Rochefort, Loranne Vernisse, Ana Cristina Gómez-Herrero, Carlos Sánchez-Sánchez, José Angel Martín-Gago, Frédéric Chérioux, Sylvain Clair, Johann Coraux, José I Martínez, Role of the Structure and Reactivity of Cu and Ag Surfaces in the Formation of a 2D Metal–Hexahydroxytriphenylene Network, Journal of Physical Chemistry C, 2021, 125, pp.17333-17341 (10.1021/acs.jpcc.1c03976) (hal-03310454) |
| Nataliya Kalashnyk, Michel Daher Mansour, Joffrey Pijeat, Rémi Plamont, Xavier Bouju, Teodor Silviu Balaban, Stéphane Campidelli, Laurence Masson, Sylvain Clair, Edge-On Self-Assembly of Tetra-bromoanthracenyl-porphyrin on Silver Surfaces, Journal of Physical Chemistry C, 2020, 124, pp.22137-22142 (10.1021/acs.jpcc.0c05908) (hal-03043738) |
| Daling Cui, Yuan Fang, Oliver Maclean, Dmitrii F. Perepichka, Federico Rosei, Sylvain Clair, Covalent organic frameworks from a monomer with reduced symmetry: polymorphism and Sierpiński triangles, Chemical Communications, 2019, 90, pp.13586-13589 (10.1039/C9CC05674B) (hal-02355234) |
| Abraham Gutierrez, Mickael Buchet, Sylvain Clair, Persistent homology to quantify the quality of surface- supported covalent networks, ChemPhysChem, 2019, 20, pp.2286-2291 (10.1002/cphc.201900257) (hal-02292844) |
| C. Pardanaud, Alexandre Merlen, K. Gratzer, Olivier Chuzel, D. Nikolaievskyi, Lionel Patrone, Sylvain Clair, R. Ramirez-Jimenez, A de Andrés, P. Roubin, Jean-Luc Parrain, Forming Weakly Interacting Multilayers of Graphene Using Atomic Force Microscope Tip Scanning and Evidence of Competition between Inner and Outer Raman Scattering Processes Piloted by Structural Defects, Journal of Physical Chemistry Letters, 2019, 10, pp.3571-3579 (10.1021/acs.jpclett.9b00564) (hal-02385952) |
| Sébastien Combes, Khoa Truong Tran, Mehmet Menaf Ayhan, Hakim Karoui, Antal Rockenbauer, Alain Tonetto, Valérie Monnier, Laurence Charles, Roselyne Rosas, Stéphane Viel, Didier Siri, Paul Tordo, Sylvain Clair, Ruibing Wang, David Bardelang, Olivier Ouari, Triangular Regulation of Cucurbit[8]uril 1:1 Complexes, Journal of the American Chemical Society, 2019, 141, pp.5897-5907 (10.1021/jacs.9b00150) (hal-02355128) |
| Sylvain Clair, Dimas de Oteyza, Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis, Chemical Reviews, 2019, 119, pp.4717-4776 (10.1021/acs.chemrev.8b00601) (hal-02095062) |
| Nataliya Kalashnyk, Eric Salomon, Sung Hwan Mun, Jaehoon Jung, Luca Giovanelli, Thierry Angot, Frédéric Dumur, Didier Gigmes, Sylvain Clair, The Orientation of Silver Surfaces Drives the Reactivity and the Selectivity in Homo-Coupling Reactions, ChemPhysChem, 2018, 19, pp.1802 - 1808 (10.1002/cphc.201800406) (hal-01896217) |
| Joffrey Pijeat, Nataliya Kalashnyk, Rémi Plamont, Teodor Silviu Balaban, Sylvain Clair, Pierre Thuéry, Stéphane Campidelli, Bottom-up synthesis of porphyrin based graphene nanoribbons and nanomeshes, International Conference on Porphyrins and Phthalocyanins, 2018 () (cea-02339594) |
| Julien Botton, Katharina Gratzer, Cyril François, Vincent Mesquita, Lionel Patrone, Teodor S Balaban, Sylvain Clair, Jean-Luc Parrain, Olivier Chuzel, Spatially resolved acyl transfer on surface by organo-catalytic scanning probe nanolithography (o-cSPL), Chemical Science, 2018, 9, pp.4280 - 4284 (10.1039/c8sc00294k) (hal-01794227) |
| Nataliya Kalashnyk, Frédéric Dumur, Didier Gigmes, Sylvain Clair, Molecular adaptation in supramolecular self-assembly: brickwall-type phases of indacene-tetrone on silver surfaces, Chemical Communications, 2018, 54, pp.8510 - 8513 (10.1039/c8cc04883e) (hal-01896219) |
| Nataliya Kalashnyk, Kawtar Mouhat, Jihun Oh, Jaehoon Jung, Yangchun Xie, Eric Salomon, Thierry Angot, Frédéric Dumur, Didier Gigmes, Sylvain Clair, On-surface synthesis of aligned functional nanoribbons monitored by scanning tunnelling microscopy and vibrational spectroscopy, Nature Communications, 2017 (10.1038/ncomms14735) (hal-01565671) |
| Manel Mabrouk, Adrien Savoyant, Luca Giovanelli, Sylvain Clair, Roland Hayn, Rafik Ben Chaabane, Ligand Influence on Local Magnetic Moments in Fe-Based Metal-Organic Networks, Journal of Physical Chemistry C, 2017, 121, pp.4253-4260 (10.1021/acs.jpcc.6b10709) (hal-01694482) |
| Manel Mabrouk, Adrien Savoyant, Luca Giovanelli, Sylvain Clair, Roland Hayn, Rafik Ben Chaabane, Ligand Influence on Local Magnetic Moments in Fe-Based Metal–Organic Networks, Journal of Physical Chemistry C, 2017, 121, pp.4253 - 4260 (10.1021/acs.jpcc.6b10709) (hal-03586844) |
| Hong-Guang Jin, Xiaoqin Jiang, Irina A. Kühne, Sylvain Clair, Valérie Monnier, Christophe Chendo, Ghenadie Novitchi, Annie K. Powell, Karl M. Kadish, Teodor Silviu Balaban, Microwave-Mediated Synthesis of Bulky Lanthanide Porphyrin–Phthalocyanine Triple-Deckers: Electrochemical and Magnetic Properties, Inorganic Chemistry, 2017, 56, pp.4864-4873 (10.1021/acs.inorgchem.6b03056) (hal-01683276) |
| Luca Giovanelli, H.-L. Lee, Corinne Lacaze-Dufaure, Mathieu Koudia, Sylvain Clair, Y.-P. Lin, Younal Ksari, Jean-Marc Themlin, Mathieu Abel, Attilio A. Cafolla, Electronic structure of tetra(4-aminophenyl)porphyrin studied by photoemission, UV–Vis spectroscopy and density functional theory, Journal of Electron Spectroscopy and Related Phenomena, 2017, 218, pp.40-45 (10.1016/j.elspec.2017.05.005) (hal-01669998) |
| Sylvain Clair, Controlling a chemical coupling reaction on a surface: a survey, OMNT International Symposium on Surface Chemistry, 2016 () (hal-01456845) |
| Vincent Mesquita, Julien Botton, Dmitry A. Valyaev, Cyril François, Lionel Patrone, Teodor Silviu Balaban, Mathieu Abel, Jean-Luc Parrain, Olivier Chuzel, Sylvain Clair, Catalytic Scanning Probe Nanolithography (cSPL): Control of the AFM Parameters in Order to Achieve Sub-100-nm Spatially Resolved Epoxidation of Alkenes Grafted onto a Surface, Langmuir, 2016, 32, pp.4034-4042 (10.1021/acs.langmuir.6b00543) (hal-01415128) |
| Sylvain Clair, Hyung-Joon Shin, Yousoo Kim, Maki Kawai, Electronic modulations in a single wall carbon nanotube induced by the Au(111) surface reconstruction, Applied Physics Letters, 2015, 106 (10.1063/1.4907613) (hal-01728978) |
| Elena Nardi, Long Chen, Sylvain Clair, Mathieu Koudia, Luca Giovanelli, Xinliang Feng, Klaus Müllen, Mathieu Abel, On-Surface Reaction between Tetracarbonitrile-Functionalized Molecules and Copper Atoms, Journal of Physical Chemistry C, 2014, 118, pp.27549 - 27553 (10.1021/jp508990s) (hal-01896213) |
| Luca Giovanelli, Oualid Ourdjini, Mathieu Abel, Rémy Pawlak, Jun Fujii, Louis Porte, Jean-Marc Themlin, Sylvain Clair, Combined Photoemission Spectroscopy and Scanning Tunneling Microscopy Study of the Sequential Dehydrogenation of Hexahydroxytriphenylene on Ag(111), Journal of Physical Chemistry C, 2014, 118, pp.14899 - 14904 (10.1021/jp501849h) (hal-01896216) |
| Shawulienu Kezilebieke, Anis Amokrane, Mauro Boero, Sylvain Clair, Mathieu Abel, Jean-Pierre Bucher, Steric and electronic selectivity in the synthesis of Fe-1,2,4,5-tetracyanobenzene (TCNB) complexes on Au(111): From topological confinement to bond formation, Nano Research, 2014, 7, pp.888 - 897 (10.1007/s12274-014-0450-y) (hal-01896211) |
| L. Giovanelli, A. Savoyant, Mathieu Abel, F. Maccherozzi, Y. Ksari, Mathieu Koudia, R. Hayn, Fadi Choueikani, E. Otero, P. Ohresser, Jean-Marc Themlin, S. Dhesi, Sylvain Clair, Magnetic Coupling and Single-Ion Anisotropy in Surface-Supported Mn-Based Metal–Organic Networks, Journal of Physical Chemistry C, 2014, 118, pp.11738 - 11744 (10.1021/jp502209q) (hal-01896214) |
| Sylvain Clair, Mathieu Abel, Louis Porte, Growth of boronic acid based two-dimensional covalent networks on a metal surface under ultrahigh vacuum, Chemical Communications, 2014, 50, pp.9627 - 9635 (10.1039/c4cc02678k) (hal-01896215) |
| Yu-Pu Lin, Oualid Ourdjini, Luca Giovanelli, Sylvain Clair, Thomas Faury, Younal Ksari, Jean-Marc Themlin, Louis Porte, Mathieu Abel, Self-Assembled Melamine Monolayer on Cu(111), Journal of Physical Chemistry C, 2013, 117, pp.9895 - 9902 (10.1021/jp401496s) (hal-01896205) |
| Dmitry A. Valyaev, Sylvain Clair, Lionel Patrone, Mathieu Abel, Louis Porte, Olivier Chuzel, Jean-Luc Parrain, Grafting a homogeneous transition metal catalyst onto a silicon AFM probe: a promising strategy for chemically constructive nanolithography, Chemical Science, 2013, 4, pp.2815-2821 (10.1039/c3sc50979f) (hal-00861617) |
| Thomas Faury, Frédéric Dumur, Sylvain Clair, Mathieu Abel, Louis Porte, Didier Gigmes, Side functionalization of diboronic acid precursors for covalent organic frameworks, CrystEngComm, 2013, 15, pp.2067-2075 (10.1039/c3ce26494g) (hal-01020191) |
| L. Giovanelli, F.C. Bocquet, P. Amsalem, L. Lee, Mathieu Abel, Sylvain Clair, Mathieu Koudia, T. Faury, L. Petaccia, D. Topwal, E. Salomon, Thierry Angot, A. Cafolla, N. Koch, L. Porte, A. Goldoni, Jean-Marc Themlin, Interpretation of valence band photoemission spectra at organic-metal interfaces, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2013, 87 (10.1103/PhysRevB.87.035413) (hal-01896208) |
| Gregory Delafosse, Alexandre Merlen, Sylvain Clair, Lionel Patrone, A Surface Enhanced Raman Spectroscopy study of aminothiophenol and aminothiophenol-C60 self-assembled monolayers: evolution of Raman modes with experimental parameters, The Journal of Chemical Physics, 2012, 136, pp.194704 (10.1063/1.4717720) (hal-01007241) |
| Sylvain Clair, Oualid Ourdjini, Mathieu Abel, Louis Porte, Two-Dimensional Polymer as a Mask for Surface Nanopatterning, Advanced Materials, 2012, 24, pp.1252 - 1254 (10.1002/adma.201200063) (hal-01896196) |
| Thomas Faury, Sylvain Clair, Mathieu Abel, Frédéric Dumur, Didier Gigmes, Louis Porte, Sequential linking to control the growth of a surface covalent organic framework, Journal of Physical Chemistry C, 2012, 116, pp.4819−4823 (10.1021/jp300417g) (hal-01020216) |
| L. Porte, Mathieu Abel, P. Amsalem, F. Bocquet, C. Bocquet, V Chevallier, Sylvain Clair, G. Delafosse, S. Desbief, Virginie Gadenne, L. Giovanelli, Mathieu Koudia, Y. Ksari, C. Loppacher, Alexandre Merlen, Laurent Nony, O. Ourdjini, Lionel Patrone, R. Pawlak, J Romann, J Valmalette, J Themlin, Self-organised growth of molecular arrays at surfaces, International Journal of Nanotechnology, 2012, 9 (10.1504/IJNT.2012.045337) (hal-01896199) |
| Sylvain Clair, Yousoo Kim, Maki Kawai, Step-edge faceting and local metallization of a single-wall semiconducting carbon nanotube, Journal of Applied Physics, 2011, 110 (10.1063/1.3646561) (hal-01896192) |
| Oualid Ourdjini, Rémy Pawlak, Mathieu Abel, Sylvain Clair, Liang Chen, Nathalie Bergeon, Michel Sassi, Vincent Oison, Jean-Marc Debierre, Roland Coratger, Louis Porte, Substrate-mediated ordering and defect analysis of a surface covalent organic framework, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2011, 84 (10.1103/PhysRevB.84.125421) (hal-01896190) |
| Sylvain Clair, Yousoo Kim, Maki Kawai, Energy level alignment of single-wall carbon nanotubes on metal surfaces, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2011, 83 (10.1103/PhysRevB.83.245422) (hal-01896194) |
| Mathieu Abel, Sylvain Clair, Oualid Ourdjini, Mireille Mossoyan, Louis Porte, Single Layer of Polymeric Fe-Phthalocyanine: An Organometallic Sheet on Metal and Thin Insulating Film, Journal of the American Chemical Society, 2011, 133, pp.1203 - 1205 (10.1021/ja108628r) (hal-01896193) |
| Sylvain Clair, Yousoo Kim, Maki Kawai, Coverage-Dependent Formation of Chiral Ethylthiolate-Au Complexes on Au(111), Langmuir, 2011, 27, pp.627 - 629 (10.1021/la103641w) (hal-01896195) |
| Sylvain Clair, Oualid Ourdjini, Mathieu Abel, Louis Porte, Tip- or electron beam-induced surface polymerization, Chemical Communications, 2011, 47 (10.1039/c1cc12065d) (hal-01896188) |
| Sylvain Clair, Mathieu Abel, Louis Porte, Mesoscopic Arrays from Supramolecular Self-Assembly, Angewandte Chemie International Edition, 2010, 49, pp.8237 - 8239 (10.1002/anie.201003335) (hal-01896187) |
| Hyung-Joon Shin, Sylvain Clair, Yousoo Kim, Maki Kawai, Substrate-induced array of quantum dots in a single-walled carbon nanotube, Nature Nanotechnology, 2009, 4, pp.567 - 570 (10.1038/NNANO.2009.182) (hal-01896184) |
| Rémy Pawlak, Sylvain Clair, Vincent Oison, Mathieu Abel, Oualid Ourdjini, Nikolas Zwaneveld, Didier Gigmes, Denis Bertin, Laurent Nony, Louis Porte, Robust Supramolecular Network on Ag(111): Hydrogen-Bond Enhancement through Partial Alcohol Dehydrogenation, ChemPhysChem, 2009, 10, pp.1032 - 1035 (10.1002/cphc.200900055) (hal-01896185) |
| Fabio Variola, Fiorenzo Vetrone, Ludovic Richert, Pawel Jedrzejowski, Ji-Hyun Yi, Sylvia Zalzal, Sylvain Clair, Andranik Sarkissian, Dmitrii F. Perepichka, James Wuest, Federico Rosei, Antonio Nanci, Improving Biocompatibility of Implantable Metals by Nanoscale Modification of Surfaces: An Overview of Strategies, Fabrication Methods, and Challenges, Small, 2009, 5, pp.996 - 1006 (10.1002/smll.200801186) (hal-01896186) |
| Pietro Gambardella, Sebastian Stepanow, Alexandre Dmitriev, Jan Honolka, Frank de Groot, Magalí Lin, Subhra Sen Gupta, D. Sarma, Peter Bencok, Stefan Stanescu, Sylvain Clair, Stéphane Pons, Nian Lin, Ari P. Seitsonen, Harald Brune, Johannes Barth, Klaus Kern, Supramolecular control of the magnetic anisotropy in two-dimensional high-spin Fe arrays at a metal interface, Nature Materials, 2009, 8, pp.189 - 193 (10.1038/NMAT2376) (hal-01896182) |
| Fabio Variola, Fiorenzo Vetrone, Ludovic Richert, Pawel Jedrzejowski, Ji-Hyun Yi, Sylvia Zalzal, Sylvain Clair, Andranik Sarkissian, Dmitrii Perepichka, James Wuest, Federico Rosei, Antonio Nanci, Improving biocompatibility of implantable metals by nanoscale modification of surfaces: an overview of strategies, fabrication methods, and challenges, Small, 2009, 5, pp.996-1006 (10.1002/smll.200801186) (hal-00667362) |
| Hyung-Joon Shin, Sylvain Clair, Yousoo Kim, Maki Kawai, Electronic structure of single-walled carbon nanotubes on ultrathin insulating films, Applied Physics Letters, 2008, 93 (10.1063/1.3046114) (hal-01896179) |
| Florian Klappenberger, Marta Cañas-Ventura, Sylvain Clair, Stéphane Pons, Uta Schlickum, Zhi-Rong Qu, Thomas Strunskus, Alessio Comisso, Christof Wöll, Harald Brune, Klaus Kern, Alessandro de Vita, Mario Ruben, Johannes Barth, Does the Surface Matter? Hydrogen-Bonded Chain Formation of an Oxalic Amide Derivative in a Two- and Three-Dimensional Environment, ChemPhysChem, 2008, 9, pp.2522 - 2530 (10.1002/cphc.200800590) (hal-01896180) |
| Sylvain Clair, Fabio Variola, Mykola Kondratenko, Pawel Jedrzejowski, Antonio Nanci, Federico Rosei, Dmitrii F. Perepichka, Self-assembled monolayer of alkanephosphoric acid on nanotextured Ti, The Journal of Chemical Physics, 2008, 128 (10.1063/1.2876421) (hal-01896178) |
| Florian Klappenberger, Marta Esther Cañas-Ventura, Sylvain Clair, Stéphane Pons, Uta Schlickum, Zhi-Rong Qu, Harald Brune, Klaus Kern, Thomas Strunskus, Christof Wöll, Alessio Comisso, Alessandro de Vita, Mario Ruben, Johannes Barth, Conformational Adaptation in Supramolecular Assembly on Surfaces, ChemPhysChem, 2007, 8, pp.1782 - 1786 (10.1002/cphc.200700370) (hal-01896161) |
| Caroline Rabot, Sylvain Clair, Yousoo Kim, Maki Kawai, Scanning Tunneling Microscopy Observations of Benzoic Acid Molecules Coadsorbed with Single-Walled Carbon Nanotubes on Au(111) surface, Japanese Journal of Applied Physics, 2007, 46, pp.5572 - 5576 (10.1143/JJAP.46.5572) (hal-01896159) |
| Sylvain Clair, Caroline Rabot, Yousoo Kim, Maki Kawai, Adsorption mechanism of aligned single wall carbon nanotubes at well defined metal surfaces, Journal of Vacuum Science & Technology B Microelectronics and Nanometer Structures, 2007, 25 (10.1116/1.2743652) (hal-01896174) |
| Marta E Cañas-Ventura, Florian Klappenberger, Sylvain Clair, Stéphane Pons, Klaus Kern, Harald Brune, Thomas Strunskus, Christof Wöll, Roman Fasel, Johannes V Barth, Coexistence of one- and two-dimensional supramolecular assemblies of terephthalic acid on Pd(111) due to self-limiting deprotonation, The Journal of Chemical Physics, 2006, 125 (10.1063/1.2364478) (hal-01896156) |
| Sylvain Clair, Stéphane Pons, Stefano Fabris, Stefano Baroni, Harald Brune, Klaus Kern, Johannes Barth, Monitoring Two-Dimensional Coordination Reactions: Directed Assembly of Co−Terephthalate Nanosystems on Au(111), Journal of Physical Chemistry B, 2006, 110, pp.5627 - 5632 (10.1021/jp057239s) (hal-01896154) |
| Sylvain Clair, Stéphane Pons, Stefano Fabris, Stefano Baroni, Harald Brune, Klaus Kern, Johannes Barth, Monitoring Two-Dimensional Coordination Reactions: Directed Assembly of Co−Terephthalate Nanosystems on Au(111), Journal of Physical Chemistry B, 2006, 110, pp.5627-5632 (10.1021/jp057239s) (hal-02997402) |
| Sylvain Clair, Stéphane Pons, Harald Brune, Klaus Kern, Johannes Barth, Mesoscopic Metallosupramolecular Texturing by Hierarchic Assembly, Angewandte Chemie International Edition, 2005, 44, pp.7294-7297 (10.1002/anie.200501906) (hal-02989405) |
| Sylvain Clair, Stéphane Pons, Harald Brune, Klaus Kern, Johannes Barth, Mesoscopic Metallosupramolecular Texturing by Hierarchic Assembly, Angewandte Chemie International Edition, 2005, 44, pp.7294 - 7297 (10.1002/anie.200501906) (hal-01896153) |
| Sylvain Clair, Stéphane Pons, Ari Seitsonen, Harald Brune, Klaus Kern, Johannes Barth, STM Study of Terephthalic Acid Self-Assembly on Au(111): Hydrogen-Bonded Sheets on an Inhomogeneous Substrate †, Journal of Physical Chemistry B, 2004, 108, pp.14585-14590 (10.1021/jp049501n) (hal-02989396) |
| Sylvain Clair, Stéphane Pons, Ari P. Seitsonen, Harald Brune, Klaus Kern, Johannes Barth, STM Study of Terephthalic Acid Self-Assembly on Au(111): Hydrogen-Bonded Sheets on an Inhomogeneous Substrate, Journal of Physical Chemistry B, 2004, 108, pp.14585 - 14590 (10.1021/jp049501n) (hal-01896152) |
| Cécile Hébert, Sylvain Clair, Christoph Eisenmenger-Sittner, Herwig Bangert, Bernard Jouffrey, Peter Schattschneider, Electron energy-loss spectroscopy fine structure of the Cu L 2,3 ionization edge in substitutional Cu-Ni alloys, Journal of Physics: Condensed Matter, 2001, 13, pp.3791 - 3803 (10.1088/0953-8984/13/16/309) (hal-01896151) |
| Andrés Lombana, Songpol Chaunchaiyakul, Olivier Chuzel, Denis Hagebaum-Reignier, Jean-Luc Parrain, Franck Bocquet, Laurent Nony, Christian Loppacher, Federica Bondino, Elena Magnano, Hiroshi Imada, Emiko Kazuma, Yousoo Kim, Luca Giovanelli, Sylvain Clair, Competing pathways to aromaticity governed by amine dehydrogenation and metal–organic complexation in on-surface synthesis, Chemical Science, 2025, 16, pp.3198 - 3210 (10.1039/d4sc07550a) (hal-04944948) |
| Laurent Nony, Sylvain Clair, Daniel Uehli, Aitziber Herrero, Jean-Marc Themlin, Andrea Campos, Franck Para, Alessandro Pioda, Christian Loppacher, Stiffness calibration of qPlus sensors at low temperature through thermal noise measurements, Beilstein Journal of Nanotechnology, 2024, 15, pp.580 - 602 (10.3762/bjnano.15.50) (hal-04729711) |
| Elie Geagea, Daniel Medina-Lopez, Luca Giovanelli, Laurent Nony, Christian Loppacher, Stéphane Campidelli, Sylvain Clair, Growth Mechanism of Chevron Graphene Nanoribbons on (111)-Oriented Coinage Metal Surfaces, Journal of Physical Chemistry C, 2024, 128, pp.8601-8610 (10.1021/acs.jpcc.4c01080) (hal-04729690) |
| Thomas Leoni, Laurent Nony, Elena Zaborova, Sylvain Clair, Frédéric Fagès, Franck Para, Alain Ranguis, Conrad Becker, Christian Loppacher, Stereoisomeric selection upon adsorption: A structural and optical study of curcuminoid derivatives on ultrathin films of KCl on Au(111) and on KCl(001) bulk, Physical Review B, 2021, 104 (10.1103/PhysRevB.104.205415) (hal-03424085) |
| Frédéric Cherioux, Laurent Nony, Franck Para, Franck Bocquet, David Gao, Filippo Federici Canova, B Watkins, Christian Loppacher, Controlling the Self-Assembly and Polymerization of Organic Molecules on Alkali Halide Surfaces, International workshop on nao- and bio-photonics, 2019 () (hal-02384106) |
| Christian Loppacher, Franck Para, Franck Bocquet, Laurent Nony, Michel Feron, Frédéric Cherioux, David Gao, Filippo Federici Canova, B Watkins, Controlling the Self-Assembly and Polymerization of Organic Molecules on Alkali Halide Surfaces, Journées des Spectroscopies d’Electrons, 2019 () (hal-02384104) |
| Christian Loppacher, F Palmino, F Chérioux, Photochemistry highlights on-surface synthesis, ChemPhysChem, 2019 () (hal-03532873) |
| Frank Palmino, Christian Loppacher, Frédéric Cherioux, Photochemistry Highlights on On‐Surface Synthesis, ChemPhysChem, 2019, 20, pp.2271 - 2280 () (hal-02366568) |
| Franck Para, Franck Bocquet, Laurent Nony, Christian Loppacher, Michel Féron, Frédéric Chérioux, David Gao, Filippo Federici Canova, Matthew B Watkins, Micrometre-long covalent organic fibres by photoinitiated chain-growth radical polymerization on an alkali-halide surface, Nature Chemistry, 2018, 10, pp.1112-1117 (10.1038/s41557-018-0120-x) (hal-01894388) |
| Franck Bocquet, Laurent Nony, Franck Para, Philipda Luangprasert, Jean-Valère Naubron, Christian Loppacher, Thomas Leoni, Anthony Thomas, Alain Ranguis, Anthony D ' Aléo, Fréderic Fagès, Conrad Becker, Noncontact AFM and differential reflectance spectroscopy joint analyses of bis-pyrenyl thin films on bulk insulators: Relationship between structural and optical properties, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2018, 97 (10.1103/physrevb.97.235434) (hal-01895225) |
| Julian Gaberle, David Z Gao, Alexander L Shluger, Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon L Lamare, Fréderic Cherioux, Morphology and Growth Mechanisms of Self-Assembled Films on Insulating Substrates: Role of Molecular Flexibility and Entropy, Journal of Physical Chemistry C, 2017, 121, pp.4393 - 4403 (10.1021/acs.jpcc.6b12738) (hal-01530418) |
| Julian Gaberle, David Gao, Alexander L Shluger, Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frédéric Chérioux, Morphology and Growth Mechanisms of Self-Assembled Films on Insulating Substrates: Role of Molecular Flexibility and Entropy, Journal of Physical Chemistry C, 2017, 121, pp.4393-4403 (10.1021/acs.jpcc.6b12738) (hal-01702376) |
| Laurent Nony, Franck Bocquet, Franck Para, Christian Loppacher, Frequency shift, damping, and tunneling current coupling with quartz tuning forks in noncontact atomic force microscopy, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2016, 94 (10.1103/PhysRevB.94.115421) (hal-01519661) |
| Laurent Nony, Franck Para, Franck Bocquet, Christian Loppacher, Integration of the RHK R9 to an Omicron VT-AFM, Application Note, 2015 () (hal-01702368) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Chérioux, David Gao, Filippo Federici Canova, Matthew B Watkins, Alexander L Shluger, Molecular Design and Control Over the Morphology of Self-Assembled Films on Ionic Substrates, Advanced Materials Interfaces, 2014, 1, pp.1400414 (10.1002/admi.201400414) (hal-01702354) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Réseaux organiques supramoléculaires étendus sur sels ioniques : Versatilité de la liaison hydrogène, Réseaux organiques supramoléculaires étendus sur sels ioniques : Versatilité de la liaison hydrogène, 2014 () (hal-00961606) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Organic Supramolecular Networks on Insulating Substrates: Versatility of the Hydrogen Bond, Organic Supramolecular Networks on Insulating Substrates: Versatility of the Hydrogen Bond, 2014 () (hal-01016773) |
| Franz Fuchs, Benjamin Grevin, Franck Bocquet, Laurent Nony, Christian Loppacher, Correct height measurements by Kelvin probe force microscopy: Poly(3-dodecylthiophene) on highly oriented pyrolytic graphite, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2013, 88 (10.1103/PhysRevB.88.205423) (hal-02277703) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941704) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941683) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941666) |
| Laurent Nony, Franck Bocquet, Frank Palmino, Frédéric Chérioux, Christian Loppacher, 2D assemblies of Zwitterionic Molecules on Alkali Halide Surfaces, Elecmol'12 Sixth International meeting on molecular eletronics, 2012 () (hal-00753655) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Christian Loppacher, Dipole-driven self-organization of zwitterionic molecules on alkali halide surfaces, International Conference on Nanoscience + Technology (ICN+T 2012), 2012 () (hal-00736815) |
| Laurent Nony, Franck Bocquet, Frédéric Chérioux, Frank Palmino, Christian Loppacher, Self-Organization of Zwitterionic Molecules on Alkali Halide Surfaces, 15th International Conference on non-contact Atomic Force Microscopy, 2012 () (hal-00736818) |
| Laurent Nony, Franck Bocquet, Frank Palmino, Frédéric Cherioux, Christian Loppacher, Self-Organization of Zwitterionic Molecules on Alkali Halide Surfaces, International Conference on Non-Contact Atomic Force Microscopy, 2012 () (hal-02300703) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Christian Loppacher, Dipole-driven self-organization of zwitterionic molecules on alkali halide surfaces, Beilstein Journal of Nanotechnology, 2012, 3, pp.285-293 (10.3762/bjnano.3.32) (hal-01702352) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Louis Porte, Christian Loppacher, The role of a molecular dipole on the adsorption on an ionic surface, 4th International Conference on non-contact Atomic Force Microscopy, 2011 () (hal-00626719) |
| Franck Bocquet, Laurent Nony, Christian Loppacher, Polarization effects in Non-Contact Atomic Force Microscopy: a key to model the tip-sample interaction above charged adatoms, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2011, 83, pp.035411 (10.1103/PhysRevB.83.035411) (hal-00559765) |
| Rémy Pawlak, Laurent Nony, Franck Bocquet, Vincent Oison, Michel Sassi, Jean-Marc Debierre, Christian Loppacher, Louis Porte, Supramolecular assemblies of 1,4-benzene diboronic acid on KCl(001), Journal of Physical Chemistry C, 2010, 114, pp.9290-9295 (10.1021/jp102044u) (hal-00487054) |
| Laurent Nony, Adam S. Foster, Franck Bocquet, Christian Loppacher, Understanding the atomic-scale contrast in Kelvin Probe Force Microscopy, Physical Review Letters, 2009, 103, pp.036802 (10.1103/PhysRevLett.103.036802) (hal-00406631) |
| Laurent Nony, Franck Bocquet, Christian Loppacher, Thilo Glatzel, On the relevance of the atomic-scale contact potential difference by Amplitude modulation- and Frequency modulation-Kelvin probe force microscopy, Nanotechnology, 2009, 20, pp.264014 (10.1088/0957-4484/20/26/264014) (hal-00401595) |
| Franck Bocquet, Laurent Nony, Christian Loppacher, Thilo Glatzel, Analytical Approach to the Local Contact Potential Difference on (001) Ionic Surfaces:~Implications for Kelvin Probe Force Microscopy, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2008, 78, pp.035410 (10.1103/PhysRevB.78.035410) (hal-00294267) |
| F.C. Bocquet, L. Giovanelli, Y. Ksari, T Ovramenko, Andrew Mayne, Gérald Dujardin, F Spillebout, Philippe Sonnet, F Bondino, Elena Magnano, Jean-Marc Themlin, Peculiar covalent bonding of C 60 / 6H-SiC(0001)-(3 × 3) probed by photoelectron spectroscopy, Journal of Physics: Condensed Matter, 2018, 30, pp.505002 (10.1088/1361-648X/aaed1a) (hal-02314067) |
| Luca Giovanelli, H.-L. Lee, Corinne Lacaze-Dufaure, Mathieu Koudia, Sylvain Clair, Y.-P. Lin, Younal Ksari, Jean-Marc Themlin, Mathieu Abel, Attilio A. Cafolla, Electronic structure of tetra(4-aminophenyl)porphyrin studied by photoemission, UV–Vis spectroscopy and density functional theory, Journal of Electron Spectroscopy and Related Phenomena, 2017, 218, pp.40-45 (10.1016/j.elspec.2017.05.005) (hal-01669998) |
| Yu-Pu Lin, Younal Ksari, Dominique Aubel, Samar Hajjar-Garreau, G. Borvon, Yohann Spiegel, Laurent Roux, Laurent Simon, Jean-Marc Themlin, Efficient and low-damage nitrogen doping of graphene via plasma-based methods, Carbon, 2015, 100, pp.337-344 (10.1016/j.carbon.2015.12.094) (cea-01338142) |
| Lionel Patrone, Grégory Delafosse, Bruno Jousselme, Yu-Pu Lin, Younal Ksari, Mathieu Abel, Mathieu Koudia, Jean-Marc Themlin, Serge Palacin, Impact of $\pi$-conjugated self-assembled monolayer structure on their electrical properties, TNT2014, 2014 () (cea-02362750) |
| Luca Giovanelli, Oualid Ourdjini, Mathieu Abel, Rémy Pawlak, Jun Fujii, Louis Porte, Jean-Marc Themlin, Sylvain Clair, Combined Photoemission Spectroscopy and Scanning Tunneling Microscopy Study of the Sequential Dehydrogenation of Hexahydroxytriphenylene on Ag(111), Journal of Physical Chemistry C, 2014, 118, pp.14899 - 14904 (10.1021/jp501849h) (hal-01896216) |
| L. Giovanelli, A. Savoyant, Mathieu Abel, F. Maccherozzi, Y. Ksari, Mathieu Koudia, R. Hayn, Fadi Choueikani, E. Otero, P. Ohresser, Jean-Marc Themlin, S. Dhesi, Sylvain Clair, Magnetic Coupling and Single-Ion Anisotropy in Surface-Supported Mn-Based Metal–Organic Networks, Journal of Physical Chemistry C, 2014, 118, pp.11738 - 11744 (10.1021/jp502209q) (hal-01896214) |
| François C. Bocquet, Régis Bisson, Jean-Marc Themlin, J.-M. Layet, Thierry Angot, Deuterium adsorption on (and desorption from) SiC(0001)-(3×3), (√3×√3)R30°, (6√3×6√3)R30° and quasi-free standing graphene obtained by hydrogen intercalation, Journal of Physics D: Applied Physics, 2014, 47, pp.094014 (10.1088/0022-3727/47/9/094014) (hal-00945850) |
| Yu-Pu Lin, Oualid Ourdjini, Luca Giovanelli, Sylvain Clair, Thomas Faury, Younal Ksari, Jean-Marc Themlin, Louis Porte, Mathieu Abel, Self-Assembled Melamine Monolayer on Cu(111), Journal of Physical Chemistry C, 2013, 117, pp.9895 - 9902 (10.1021/jp401496s) (hal-01896205) |
| L. Giovanelli, F.C. Bocquet, P. Amsalem, L. Lee, Mathieu Abel, Sylvain Clair, Mathieu Koudia, T. Faury, L. Petaccia, D. Topwal, E. Salomon, Thierry Angot, A. Cafolla, N. Koch, L. Porte, A. Goldoni, Jean-Marc Themlin, Interpretation of valence band photoemission spectra at organic-metal interfaces, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2013, 87 (10.1103/PhysRevB.87.035413) (hal-01896208) |
| François C. Bocquet, Régis Bisson, Jean-Marc Themlin, J.-M. Layet, Thierry Angot, Reversible Hydrogenation of Deuterium-Intercalated Quasi-Free-Standing Graphene on SiC(0001), Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2012, 85, pp.201401(R) (10.1103/PhysRevB.85.201401) (hal-00834745) |
| Éric Duverger, J.-C. Labrune, Gwénaël Rapenne, M. Mossoyan-Deneux, Jean-Marc Themlin, F. Palmino, Self-alignment molecular chains on pre-structured Sm/Si(111) interfaces, 2006, pp.xx () (hal-00091922) |
| Anne Charrier, Alessandro Coati, T. Argunova, Franck Thibaudau, Y. Garreau, R. Pinchaux, I. Forbeaux, J.-M. Debever, M. Sauvage-Simkin, Jean-Marc Themlin, Solid-state decomposition of silicon carbide for growing ultra-thin heteroepitaxial graphite films, Journal of Applied Physics, 2002, 92, pp.2479 - 2484 (10.1063/1.1498962) (hal-01900009) |
| Anne Charrier, R. Pérez, Franck Thibaudau, J.-M. Debever, J. Ortega, F. Flores, Jean-Marc Themlin, Contrasted electronic properties of Sn-adatom-based ( 3 × 3 ) R 30 ° reconstructions on Si(111), Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2001, 64 (10.1103/physrevb.64.115407) (hal-01900001) |
| Jean-Marc Themlin, J.-M. Gilles, R. Johnson, Oxygen 2s spectroscopy of tin oxides with synchrotron radiation-induced photoemission, Journal de Physique IV Proceedings, 1994, 04, pp.C9-183-C9-186 (10.1051/jp4:1994931) (jpa-00253492) |
| Andrés Lombana, Songpol Chaunchaiyakul, Olivier Chuzel, Denis Hagebaum-Reignier, Jean-Luc Parrain, Franck Bocquet, Laurent Nony, Christian Loppacher, Federica Bondino, Elena Magnano, Hiroshi Imada, Emiko Kazuma, Yousoo Kim, Luca Giovanelli, Sylvain Clair, Competing pathways to aromaticity governed by amine dehydrogenation and metal–organic complexation in on-surface synthesis, Chemical Science, 2025, 16, pp.3198 - 3210 (10.1039/d4sc07550a) (hal-04944948) |
| Laurent Nony, Sylvain Clair, Daniel Uehli, Aitziber Herrero, Jean-Marc Themlin, Andrea Campos, Franck Para, Alessandro Pioda, Christian Loppacher, Stiffness calibration of qPlus sensors at low temperature through thermal noise measurements, Beilstein Journal of Nanotechnology, 2024, 15, pp.580 - 602 (10.3762/bjnano.15.50) (hal-04729711) |
| Elie Geagea, Daniel Medina-Lopez, Luca Giovanelli, Laurent Nony, Christian Loppacher, Stéphane Campidelli, Sylvain Clair, Growth Mechanism of Chevron Graphene Nanoribbons on (111)-Oriented Coinage Metal Surfaces, Journal of Physical Chemistry C, 2024, 128, pp.8601-8610 (10.1021/acs.jpcc.4c01080) (hal-04729690) |
| Clément Antoine Reynaud, David Duche, Judikaël Le Rouzo, Antoine Nasser, Laurent Nony, Florent Pourcin, O. Margeat, Jörg Ackermann, Gérard Berginc, Christian Nijhuis, Ludovic Escoubas, Jean-Jacques Simon, Enhancing Reproducibility and Nonlocal Effects in Film-Coupled Nanoantennas, Advanced Optical Materials, 2018, 6, pp.1801177 (10.1002/adom.201801177) (hal-02135960) |
| Franck Para, Franck Bocquet, Laurent Nony, Christian Loppacher, Michel Féron, Frédéric Chérioux, David Gao, Filippo Federici Canova, Matthew B Watkins, Micrometre-long covalent organic fibres by photoinitiated chain-growth radical polymerization on an alkali-halide surface, Nature Chemistry, 2018, 10, pp.1112-1117 (10.1038/s41557-018-0120-x) (hal-01894388) |
| Franck Bocquet, Laurent Nony, Franck Para, Philipda Luangprasert, Jean-Valère Naubron, Christian Loppacher, Thomas Leoni, Anthony Thomas, Alain Ranguis, Anthony D ' Aléo, Fréderic Fagès, Conrad Becker, Noncontact AFM and differential reflectance spectroscopy joint analyses of bis-pyrenyl thin films on bulk insulators: Relationship between structural and optical properties, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2018, 97 (10.1103/physrevb.97.235434) (hal-01895225) |
| Julian Gaberle, David Z Gao, Alexander L Shluger, Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon L Lamare, Fréderic Cherioux, Morphology and Growth Mechanisms of Self-Assembled Films on Insulating Substrates: Role of Molecular Flexibility and Entropy, Journal of Physical Chemistry C, 2017, 121, pp.4393 - 4403 (10.1021/acs.jpcc.6b12738) (hal-01530418) |
| D. Gao, J. Gaberle, A. Shluger, A. Amrous, F. Bocquet, Franck Para, Laurent Nony, Ch. Loppacher, S. Lamare, F. Palmino, F. Cherioux, Dynamic Effects: Adsorption of Organic Molecules at Insulating Surfaces, 19th International conference on nc-AFM, 2016 () (hal-01456850) |
| Laurent Nony, Principle of KPFM and applications, 2nd German-French summer school on nc-AFM, 2016 () (hal-01456849) |
| Laurent Nony, Franck Bocquet, Franck Para, Christian Loppacher, Frequency shift, damping, and tunneling current coupling with quartz tuning forks in noncontact atomic force microscopy, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2016, 94 (10.1103/PhysRevB.94.115421) (hal-01519661) |
| Laurent Nony, Franck Para, Franck Bocquet, Christian Loppacher, Integration of the RHK R9 to an Omicron VT-AFM, Application Note, 2015 () (hal-01702368) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Chérioux, David Gao, Filippo Federici Canova, Matthew B Watkins, Alexander L Shluger, Molecular Design and Control Over the Morphology of Self-Assembled Films on Ionic Substrates, Advanced Materials Interfaces, 2014, 1, pp.1400414 (10.1002/admi.201400414) (hal-01702354) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Réseaux organiques supramoléculaires étendus sur sels ioniques : Versatilité de la liaison hydrogène, Réseaux organiques supramoléculaires étendus sur sels ioniques : Versatilité de la liaison hydrogène, 2014 () (hal-00961606) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Organic Supramolecular Networks on Insulating Substrates: Versatility of the Hydrogen Bond, Organic Supramolecular Networks on Insulating Substrates: Versatility of the Hydrogen Bond, 2014 () (hal-01016773) |
| R. Boubekri, E. Cambril, L. Couraud, L. Bernardi, A. Madouri, Marc Portail, T. Chassagne, C. Moisson, M. Zielinski, Sai Jiao, Jean-François Michaud, Daniel Alquier, J. Bouloc, Laurent Nony, F. Bocquet, C. Loppacher, David Martrou, Sebastien Gauthier, Electrothermally driven high-frequency piezoresistive SiC cantilevers for dynamic atomic force microscopy, Journal of Applied Physics, 2014, 116, pp.054304 (10.1063/1.4891833) (hal-01715334) |
| Franz Fuchs, Benjamin Grevin, Franck Bocquet, Laurent Nony, Christian Loppacher, Correct height measurements by Kelvin probe force microscopy: Poly(3-dodecylthiophene) on highly oriented pyrolytic graphite, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2013, 88 (10.1103/PhysRevB.88.205423) (hal-02277703) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941704) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941683) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941666) |
| Fabien Castanié, Laurent Nony, Sebastien Gauthier, Xavier Bouju, Image calculations with a numerical frequency-modulation atomic force microscope, Journal of Physical Chemistry C, 2013, 117, pp.10492-10501 (10.1021/jp400948a) (hal-01797570) |
| Laurent Nony, Franck Bocquet, Frank Palmino, Frédéric Chérioux, Christian Loppacher, 2D assemblies of Zwitterionic Molecules on Alkali Halide Surfaces, Elecmol'12 Sixth International meeting on molecular eletronics, 2012 () (hal-00753655) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Christian Loppacher, Dipole-driven self-organization of zwitterionic molecules on alkali halide surfaces, International Conference on Nanoscience + Technology (ICN+T 2012), 2012 () (hal-00736815) |
| Laurent Nony, Franck Bocquet, Frédéric Chérioux, Frank Palmino, Christian Loppacher, Self-Organization of Zwitterionic Molecules on Alkali Halide Surfaces, 15th International Conference on non-contact Atomic Force Microscopy, 2012 () (hal-00736818) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Christian Loppacher, Dipole-driven self-organization of zwitterionic molecules on alkali halide surfaces, Beilstein Journal of Nanotechnology, 2012, 3, pp.285-293 (10.3762/bjnano.3.32) (hal-01702352) |
| L. Porte, Mathieu Abel, P. Amsalem, F. Bocquet, C. Bocquet, V Chevallier, Sylvain Clair, G. Delafosse, S. Desbief, Virginie Gadenne, L. Giovanelli, Mathieu Koudia, Y. Ksari, C. Loppacher, Alexandre Merlen, Laurent Nony, O. Ourdjini, Lionel Patrone, R. Pawlak, J Romann, J Valmalette, J Themlin, Self-organised growth of molecular arrays at surfaces, International Journal of Nanotechnology, 2012, 9 (10.1504/IJNT.2012.045337) (hal-01896199) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Louis Porte, Christian Loppacher, The role of a molecular dipole on the adsorption on an ionic surface, 4th International Conference on non-contact Atomic Force Microscopy, 2011 () (hal-00626719) |
| Franck Bocquet, Laurent Nony, Christian Loppacher, Polarization effects in Non-Contact Atomic Force Microscopy: a key to model the tip-sample interaction above charged adatoms, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2011, 83, pp.035411 (10.1103/PhysRevB.83.035411) (hal-00559765) |
| Rémy Pawlak, Laurent Nony, Franck Bocquet, Vincent Oison, Michel Sassi, Jean-Marc Debierre, Christian Loppacher, Louis Porte, Supramolecular assemblies of 1,4-benzene diboronic acid on KCl(001), Journal of Physical Chemistry C, 2010, 114, pp.9290-9295 (10.1021/jp102044u) (hal-00487054) |
| Laurent Nony, Adam S. Foster, Franck Bocquet, Christian Loppacher, Understanding the atomic-scale contrast in Kelvin Probe Force Microscopy, Physical Review Letters, 2009, 103, pp.036802 (10.1103/PhysRevLett.103.036802) (hal-00406631) |
| Laurent Nony, Franck Bocquet, Christian Loppacher, Thilo Glatzel, On the relevance of the atomic-scale contact potential difference by Amplitude modulation- and Frequency modulation-Kelvin probe force microscopy, Nanotechnology, 2009, 20, pp.264014 (10.1088/0957-4484/20/26/264014) (hal-00401595) |
| Rémy Pawlak, Sylvain Clair, Vincent Oison, Mathieu Abel, Oualid Ourdjini, Nikolas Zwaneveld, Didier Gigmes, Denis Bertin, Laurent Nony, Louis Porte, Robust Supramolecular Network on Ag(111): Hydrogen-Bond Enhancement through Partial Alcohol Dehydrogenation, ChemPhysChem, 2009, 10, pp.1032 - 1035 (10.1002/cphc.200900055) (hal-01896185) |
| Franck Bocquet, Laurent Nony, Christian Loppacher, Thilo Glatzel, Analytical Approach to the Local Contact Potential Difference on (001) Ionic Surfaces:~Implications for Kelvin Probe Force Microscopy, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2008, 78, pp.035410 (10.1103/PhysRevB.78.035410) (hal-00294267) |
| Laurent Nony, Alexis Baratoff, Dominique Schaer, Oliver Pfeiffer, Adrian Wezel, Ernst Meyer, Noncontact atomic force microscopy simulator with phase-locked-loop controlled frequency detection and excitation, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2006, 74, pp.235439 (10.1103/PhysRevB.74.235439) (hal-00124764) |
| Oliver Pfeiffer, Laurent Nony, Roland Bennewitz, Alexis Baratoff, Ernst Meyer, Distance dependence of force and dissipation in non-contact atomic force microscopy on Cu(100) and Al(111), Nanotechnology, 2004, 15, pp.S101-S107 (10.1088/0957-4484/15/2/021) (hal-00014042) |
| Laurent Nony, Enrico Gnecco, Alexis Baratoff, Audrius Alkauskas, Roland Bennewitz, Oliver Pfeiffer, Sabine Maier, Adrian Wezel, Ernst Meyer, Christophe Gerber, Observation of individual molecules trapped on a nanostructured insulator, Nano Letters, 2004, 4, pp.2185-2189 (10.1021/nl048693v) (hal-00014222) |
| Laurent Nony, Roland Bennewitz, Oliver Pfeiffer, Enrico Gnecco, Alexis Baratoff, Ernst Meyer, Toyoaki Eguchi, André Gourdon, Christian Joachim, Cu-TBPP and PTCDA molecules on insulating surfaces studied by ultra-high-vacuum non-contact AFM, Nanotechnology, 2004, 15, pp.S91-S96 (10.1088/0957-4484/15/2/019) (hal-00014040) |
| Gérard Couturier, Rodolphe Boisgard, Laurent Nony, Jean-Pierre Aimé, Non-contact atomic force microscopy: Stability criterion and dynamical responses of the shift of frequency and damping signal, Review of Scientific Instruments, 2003, 74, pp.2726-2734 (10.1063/1.1564274) (hal-00012403) |
| Gérard Couturier, Laurent Nony, Rodolphe Boisgard, Jean-Pierre Aimé, Stability analysis of an oscillating tip-cantilever system in NC-AFM, Applied Surface Science, 2002, 188, pp.341-348 () (hal-00012047) |
| Laurent Nony, Touria Bouhacina, Jean-Pierre Aimé, Dissipation induced by attractive interaction in dynamic force microscopy : contribution of adsorbed water layers, Surface Science : A Journal Devoted to the Physics and Chemistry of Interfaces, 2002, 499, pp.152-160 () (hal-00004589) |
| H. Nanjo, Laurent Nony, M. Yoneya, J. P. Aime, Simulation of section curve by phase constant dynamic mode atomic force microscopy in non-contact situation, 5th International Conference on Noncontract Atomic Force Microscopy (NC-AFM 2002), 2002, pp.49-53 (10.1016/s0169-4332(02)01478-2) (hal-01550775) |
| Laurent Nony, T. Cohen-Bouhacina, J. P. Aime, Dissipation induced by attractive interaction in dynamic force microscopy: contribution of adsorbed water layers, Surface Science : A Journal Devoted to the Physics and Chemistry of Interfaces, 2002, 499, pp.152-160 () (hal-01550777) |
| Gérard Couturier, Laurent Nony, Rodolphe Boisgard, Jean-Pierre Aimé, Stability of an oscillating tip in Non-Contact Atomic Force Microscopy: theoretical and numerical investigations., Applied Physics, 2002, 91 (4), pp.2537-2543 () (hal-00004588) |
| G. Couturier, Laurent Nony, R. Boisgard, J. P. Aime, Stability of an oscillating tip in noncontact atomic force microscopy: Theoretical and numerical investigations, Journal of Applied Physics, 2002, 91, pp.2537-2543 () (hal-01550829) |
| S. Navarre, F. Choplin, J. Bousbaa, B. Bennetau, Laurent Nony, J. P. Aime, Structural characterization of self-assembled monolayers of organosilanes chemically bonded onto silica wafers by dynamical force microscopy, Langmuir, 2001, 17, pp.4844-4850 () (hal-01550662) |
| H. Nanjo, Laurent Nony, M. Yoneya, N. Sanada, T. Iijima, J. P. Aime, Simulation of fluctuation and dissipation in dynamic force microscopy, 4th International Conference on Noncontact Atomic Force Microscopy, 2001, pp.349-354 () (hal-01550659) |
| G. Couturier, Laurent Nony, R. Boisgard, J. P. Aime, Stability analysis of an oscillating tip-cantilever system in NC-AFM, 4th International Conference on Noncontact Atomic Force Microscopy, 2001, pp.341-348 () (hal-01550696) |
| Laurent Nony, Rodolphe Boisgard, Jean-Pierre Aimé, DNA properties investigated by dynamic force microscopy, Biomacromolecules, 2001, 2, pp.827-835 () (hal-00004532) |
| Laurent Nony, Rodolphe Boisgard, Jean-Pierre Aimé, Stability criterions of an oscillating tip-cantilever system in dynamic force microscopy, The European Physical Journal B: Condensed Matter and Complex Systems, 2001, 24, pp.221-229 () (hal-00004544) |
| Jean-Pierre Aimé, Rodolphe Boisgard, Laurent Nony, Gérard Couturier, Influence of noncontact dissipation in the tapping mode: Attempt to extract quantitative information on the surface properties with the local force probe method, The Journal of Chemical Physics, 2001, 114, pp.4945-4954 (10.1063/1.1349179) (hal-00008335) |
| Sébastien Navarre, Franck Choplin, J. Bousbaa, Bernard Bennetau, Laurent Nony, Jean-Pierre Aimé, Structural Characterization of Self-Assembled Monolayers of Organosilanes Chemically Bonded on Silica Wafers by Dynamical Force Microscopy, Langmuir, 2001, 17, pp.4844-4850 () (hal-00004533) |
| Jean-Pierre Aimé, Gérard Couturier, Rodolphe Boisgard, Laurent Nony, Relationship between the non linear dynamic behaviour of an oscillating tip-microlever system and the contrast at the atomic scale, Applied Surface Science, 1999, 140, pp.333-338 () (hal-00011235) |
| Laurent Nony, Rodolphe Boisgard, Jean-Pierre Aimé, Nonlinear dynamical properties of an oscillating tip-cantilever system in the tapping mode, The Journal of Chemical Physics, 1999, 111(4), pp.1615-1627 (10.1063/1.479422) (hal-00011145) |
| Jean-Pierre Aimé, Denis Michel, Rodolphe Boisgard, Laurent Nony, Growth kinetics of a nanoprotuberance under the action of an oscillating nanotip, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 1999, 59, pp.2407 () (hal-00011214) |
| Jean-Pierre Aimé, Rodolphe Boisgard, Laurent Nony, Gérard Couturier, Nonlinear Dynamic Behavior of an Oscillating Tip-Microlever System and Contrast at the Atomic Scale, Physical Review Letters, 1999, 82(17), pp.3388-3391 () (hal-00011230) |
| J. P. Aime, G. Couturier, R. Boisgard, Laurent Nony, Relationship between the non linear dynamic behaviour of an oscillating tip-microlever system and the contrast at the atomic scale, 1st International Workshop on Noncontact Atomic Force Microscopy (NC-AFM 98), 1998, pp.333-338 () (hal-01550297) |
| Laurent Nony, Sylvain Clair, Daniel Uehli, Aitziber Herrero, Jean-Marc Themlin, Andrea Campos, Franck Para, Alessandro Pioda, Christian Loppacher, Stiffness calibration of qPlus sensors at low temperature through thermal noise measurements, Beilstein Journal of Nanotechnology, 2024, 15, pp.580 - 602 (10.3762/bjnano.15.50) (hal-04729711) |
| Franck Para, Franck Bocquet, Laurent Nony, Christian Loppacher, Michel Féron, Frédéric Chérioux, David Gao, Filippo Federici Canova, Matthew B Watkins, Micrometre-long covalent organic fibres by photoinitiated chain-growth radical polymerization on an alkali-halide surface, Nature Chemistry, 2018, 10, pp.1112-1117 (10.1038/s41557-018-0120-x) (hal-01894388) |
| Franck Bocquet, Laurent Nony, Franck Para, Philipda Luangprasert, Jean-Valère Naubron, Christian Loppacher, Thomas Leoni, Anthony Thomas, Alain Ranguis, Anthony D ' Aléo, Fréderic Fagès, Conrad Becker, Noncontact AFM and differential reflectance spectroscopy joint analyses of bis-pyrenyl thin films on bulk insulators: Relationship between structural and optical properties, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2018, 97 (10.1103/physrevb.97.235434) (hal-01895225) |
| Julian Gaberle, David Z Gao, Alexander L Shluger, Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon L Lamare, Fréderic Cherioux, Morphology and Growth Mechanisms of Self-Assembled Films on Insulating Substrates: Role of Molecular Flexibility and Entropy, Journal of Physical Chemistry C, 2017, 121, pp.4393 - 4403 (10.1021/acs.jpcc.6b12738) (hal-01530418) |
| Julian Gaberle, David Gao, Alexander L Shluger, Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frédéric Chérioux, Morphology and Growth Mechanisms of Self-Assembled Films on Insulating Substrates: Role of Molecular Flexibility and Entropy, Journal of Physical Chemistry C, 2017, 121, pp.4393-4403 (10.1021/acs.jpcc.6b12738) (hal-01702376) |
| D. Gao, J. Gaberle, A. Shluger, A. Amrous, F. Bocquet, Franck Para, Laurent Nony, Ch. Loppacher, S. Lamare, F. Palmino, F. Cherioux, Dynamic Effects: Adsorption of Organic Molecules at Insulating Surfaces, 19th International conference on nc-AFM, 2016 () (hal-01456850) |
| Laurent Nony, Franck Bocquet, Franck Para, Christian Loppacher, Frequency shift, damping, and tunneling current coupling with quartz tuning forks in noncontact atomic force microscopy, Physical Review B: Condensed Matter and Materials Physics (1998-2015), 2016, 94 (10.1103/PhysRevB.94.115421) (hal-01519661) |
| Laurent Nony, Franck Para, Franck Bocquet, Christian Loppacher, Integration of the RHK R9 to an Omicron VT-AFM, Application Note, 2015 () (hal-01702368) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Chérioux, David Gao, Filippo Federici Canova, Matthew B Watkins, Alexander L Shluger, Molecular Design and Control Over the Morphology of Self-Assembled Films on Ionic Substrates, Advanced Materials Interfaces, 2014, 1, pp.1400414 (10.1002/admi.201400414) (hal-01702354) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Réseaux organiques supramoléculaires étendus sur sels ioniques : Versatilité de la liaison hydrogène, Réseaux organiques supramoléculaires étendus sur sels ioniques : Versatilité de la liaison hydrogène, 2014 () (hal-00961606) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Organic Supramolecular Networks on Insulating Substrates: Versatility of the Hydrogen Bond, Organic Supramolecular Networks on Insulating Substrates: Versatility of the Hydrogen Bond, 2014 () (hal-01016773) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941704) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941683) |
| Ania Amrous, Franck Bocquet, Laurent Nony, Franck Para, Christian Loppacher, Simon Lamare, Frank Palmino, Frédéric Cherioux, David Gao, Matt Wattkins, Alex Shluger, Controlling the adsorption of specially designed molecules for ionic substrates, Controlling the adsorption of specially designed molecules for ionic substrates, 2013 () (hal-00941666) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Christian Loppacher, Dipole-driven self-organization of zwitterionic molecules on alkali halide surfaces, International Conference on Nanoscience + Technology (ICN+T 2012), 2012 () (hal-00736815) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Christian Loppacher, Dipole-driven self-organization of zwitterionic molecules on alkali halide surfaces, Beilstein Journal of Nanotechnology, 2012, 3, pp.285-293 (10.3762/bjnano.3.32) (hal-01702352) |
| Laurent Nony, Franck Bocquet, Franck Para, Frédéric Chérioux, Éric Duverger, Frank Palmino, Vincent Luzet, Louis Porte, Christian Loppacher, The role of a molecular dipole on the adsorption on an ionic surface, 4th International Conference on non-contact Atomic Force Microscopy, 2011 () (hal-00626719) |
| Etienne Samain, Jonathan Weick, Patrick Vrancken, Franck Para, Dominique Albanese, Jocelyn Paris, Jean-Marie Torre, Cheng Zhao, Philippe Guillemot, Isabelle Petitbon, TIME TRANSFER BY LASER LINK − THE T2L2 EXPERIMENT ON JASON-2 AND FURTHER EXPERIMENTS, International Journal of Modern Physics D, 2008, 17, pp.1043 - 1054 (10.1142/S0218271808012681) (hal-01702334) |
| Junlong Wang, Virginie Gadenne, Lionel Patrone, J.M. Raimundo, Self-assembled monolayers of push-pull chromophores as active layer and their applications, Molecules, 2024, 29, pp.559 (10.3390/molecules29030559) (hal-04787744) |
| Martin Hruška, Joris More-Chevalier, Přemysl Fitl, Michal Novotný, Petr Hruška, Dejan Prokop, Petr Pokorný, Jan Kejzlar, Virginie Gadenne, Lionel Patrone, Martin Vrňata, Jan Lančok, Surface Enhancement Using Black Coatings for Sensor Applications, Nanomaterials, 2022, 12, pp.4297 (10.3390/nano12234297) (hal-04046665) |
| Estéban Sanchez-Adaime, D. Duché, Stephanie Escoubas, V. Jangid, L. Nony, A. Moreau, J. Lumeau, Lionel Patrone, C. Lebouin, L. Escoubas, Atomic force microscopy and in situ-annealing X-ray diffraction study on template-stripped gold substrates for optimum self-assembled monolayer deposition, Thin Solid Films, 2021, 739, pp.138978 (10.1016/j.tsf.2021.138978) (hal-03436579) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Hela Mrezguia, Luca Giovanelli, Younal Ksari, Victorien Jeux, Guillaume Monier, J.M. Raimundo, Lionel Patrone, Structural and electrical properties of self-assembled monolayers on germanium as passivating and insulating layers, 16th European Vacuum Conference (EVC 16), 2021 () (hal-03611144) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Hela Mrezguia, Luca Giovanelli, Younal Ksari, Victorien Jeux, G. Monier, J.M. Raimundo, Lionel Patrone, Formation, structure, and electrical properties of self-assembled monolayers on Ge as passivating and insulating layers, 13th International Conference on Physics of Advanced Materials (ICPAM-13), 2021 () (hal-03611131) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Hela Mrezguia, Luca Giovanelli, Younal Ksari, Victorien Jeux, Guillaume Monier, J.M. Raimundo, Lionel Patrone, Structural & electrical properties of self-assembled organic monolayers on germanium as passivating and insulating films, 12th Meeting on Nanoscience Advance, 2021 () (hal-03611149) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Hela Mrezguia, Luca Giovanelli, Younal Ksari, Victorien Jeux, Guillaume Monier, J.M. Raimundo, Lionel Patrone, Propriétés structurales et électriques de monocouches auto-assemblées passivantes et isolantes sur germanium, 17èmes Journées de la Matière Condensé, 2021 () (hal-03611147) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Hela Mrezguia, Luca Giovanelli, Younal Ksari, Victorien Jeux, Guillaume Monier, J.M. Raimundo, Lionel Patrone, Structural and electrical properties of self-assembled monolayers on germanium as passivating and insulating layers, Materials Research Society virtual Spring meeting, 2021 () (hal-03611145) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Younal Ksari, David Tomecek, Martin Hruska, Premysl Fitl, J.M. Raimundo, Lionel Patrone, Structural and Electrical Properties of Self-Assembled Molecular Nanodielectrics on Germanium, Electronic Materials conference, MRS, 2020 () (hal-02885477) |
| Yannick Dufil, Virginie Gadenne, Pascal Carrière, Jean-Michel Nunzi, Lionel Patrone, Growth and organization of (3-Trimethoxysilylpropyl) diethylenetriamine within reactive amino-terminated self-assembled monolayer on silica, Applied Surface Science, 2020, 508, pp.145210 (10.1016/j.apsusc.2019.145210) (hal-03048774) |
| Virginie Gadenne, B. Grenier, C. Praveen, Philippe Marsal, J Valmalette, Lionel Patrone, J. M Raimundo, Combined SERS/DFT studies of push–pull chromophore self-assembled monolayers: insights into their surface orientation, Physical Chemistry Chemical Physics, 2019, 21, pp.25865-25871 (10.1039/C9CP04008K) (hal-02396854) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Younal Ksari, J.M. Raimundo, Lionel Patrone, Fonctionnalisation du Ge par des monocouches auto-assemblées : application aux nanodiélectriques, SPIC 2019, Science et Technologie des Systèmes pi-Conjugués, 2019 () (hal-02374834) |
| C. Pardanaud, Alexandre Merlen, K. Gratzer, Olivier Chuzel, D. Nikolaievskyi, Lionel Patrone, Sylvain Clair, R. Ramirez-Jimenez, A de Andrés, P. Roubin, Jean-Luc Parrain, Forming Weakly Interacting Multilayers of Graphene Using Atomic Force Microscope Tip Scanning and Evidence of Competition between Inner and Outer Raman Scattering Processes Piloted by Structural Defects, Journal of Physical Chemistry Letters, 2019, 10, pp.3571-3579 (10.1021/acs.jpclett.9b00564) (hal-02385952) |
| Mohamed-Amine Guerboukha, Edward Kionga-Kamau, Virginie Gadenne, Younal Ksari, Bruno Jousselme, J.M. Raimundo, Lionel Patrone, Nanodiélectriques moléculaires auto-assemblés sur germanium, Forum des microscopies à sondes locales, 2019 () (hal-02157265) |
| Mohamed-Amine Guerboukha, Virginie Gadenne, Younal Ksari, David Tomecek, Martin Hruska, Jan Vlcek, Premysl Fitl, Martin Vrnata, J.M. Raimundo, Lionel Patrone, Towards self-assembled nanodielectrics on germanium, 21èmes Journées Nationales du Réseau Doctoral en Micro-Nanoélectronique, JNRDM 2019, 2019 () (hal-02157264) |
| Julien Botton, Katharina Gratzer, Cyril François, Vincent Mesquita, Lionel Patrone, Teodor S Balaban, Sylvain Clair, Jean-Luc Parrain, Olivier Chuzel, Spatially resolved acyl transfer on surface by organo-catalytic scanning probe nanolithography (o-cSPL), Chemical Science, 2018, 9, pp.4280 - 4284 (10.1039/c8sc00294k) (hal-01794227) |
| David Tomecek, Martin Hruska, Premysl Fitl, Jan Vlcek, Eva Maresova, Sarka Havlova, Lionel Patrone, Martin Vrnata, Phthalocyanine Photoregeneration for Low Power Consumption Chemiresistors, ACS Sensors, 2018, 3, pp.2558-2565 (10.1021/acssensors.8b00922) (hal-02117021) |
| C. Reynaud, D. Duche, Carmen M. Ruiz, U. Palanchoke, Lionel Patrone, J. Le Rouzo, S. Labau, N. Frolet, C. Gourgon, Claude Alfonso, A. Charai, C. Lebouin, J-J. Simon, Ludovic Escoubas, Toward a nanoimprinted nanoantenna to perform optical rectification through molecular diodes, Journal of Nanoparticle Research, 2017, 19 (10.1007/s11051-017-4091-4) (hal-01738066) |
| Lionel Patrone, Volodymyr Malytskyi, Virginie Gadenne, Grégory Delafosse, Simon Desbief, Younal Ksari, J.M. Raimundo, Jean Jacques Simon, David Duché, Ludovic Escoubas, Bruno Jousselme, Self−assembled monolayers of small organic molecules for tuning the electrical and optical properties of surfaces, 4th International Workshop on Nano and Bio−Photonics, 2017 () (hal-03611137) |
| Lionel Patrone, V. Malytskyi, Virginie Gadenne, G. Delafosse, S. Desbief, J.M. Raimundo, J.J. Simon, Bruno Jousselme, Dominique Vuillaume, Self-assembled monolayers as a smart strategy to arrange electro- and photo-active small organic molecules at surfaces, Baltic Polymer Symposium 2017, 2017 () (cea-02340937) |
| Volodymyr Malytskyi, Virginie Gadenne, Younal Ksari, Lionel Patrone, J.M. Raimundo, Synthesis and characterization of thiophene-based push-pull chromophores for tuning the electrical and optical properties of surfaces with controlled SAM formation, Tetrahedron, 2017, 73, pp.5738-5744 (10.1016/j.tet.2017.08.004) (hal-01794687) |
| C. Reynaud, D. Duche, U. Palanchoke, F-X. Dang, Lionel Patrone, J. Le Rouzo, Ludovic Escoubas, C. Gourgon, A. Charai, Claude Alfonso, O. Margeat, Jörg Ackermann, S. Balaban, J-J. Simon, Harvesting light energy with optical rectennas, TechConnect Briefs, 2017, pp.45-48 () (hal-01825527) |
| Lionel Patrone, Self−assembled monolayers as a smart strategy to arrange electro− and photo−active organic molecules at surfaces, EMN (Energy Material & Nanotechnology): Meeting on Smart & Multifunctional Materials, 2016 () (hal-01456842) |
| Virginie Gadenne, L. Fillaud, Bruno Jousselme, V. Malytskyi, J.M. Raimundo, Lionel Patrone, Passivation and electronic properties of germanium functionalized by self−assembled monolayers, Conférence Nationale sur les Processus ULtimes d’épitaxie des SEmiconducteurs, 2016 () (hal-01456876) |
| V. Malytskyi, J.M. Raimundo, J.J. Simon, Lionel Patrone, Self−assembled monolayers as a strategy to organize novel thiophene−based push−pull chromophores for electro−optical applications, NanoSEA (Nanostructures and Nanomaterials Self−Assembly), 2016 () (hal-01456853) |
| Virginie Gadenne, G. Delafosse, L. Fillaud, Bruno Jousselme, V. Malytskyi, J.M. Raimundo, Lionel Patrone, Passivation of Ge and GaAs with self−assembled organic monolayers, NanoSEA (Nanostructures and Nanomaterials Self−Assembly), 2016 () (hal-01456858) |
| Lionel Patrone, Self−assembly as a smart strategy to arrange electro− and photo−active organic molecules at surfaces, Conference proceedings of ETCMOS (Emerging Technologies, Communications, Microsystems, Optoelectronics, Sensors), 2016 () (hal-01456834) |
| Lionel Patrone, V. Malytskyi, J.J. Simon, J.M. Raimundo, Nouveaux chromophores push−pull à base de thiophène : synthèse et monocouches auto−assemblées, Forum des microscopies à sondes locales, 2016 () (hal-01456887) |
| Vincent Mesquita, Julien Botton, Dmitry A. Valyaev, Cyril François, Lionel Patrone, Teodor Silviu Balaban, Mathieu Abel, Jean-Luc Parrain, Olivier Chuzel, Sylvain Clair, Catalytic Scanning Probe Nanolithography (cSPL): Control of the AFM Parameters in Order to Achieve Sub-100-nm Spatially Resolved Epoxidation of Alkenes Grafted onto a Surface, Langmuir, 2016, 32, pp.4034-4042 (10.1021/acs.langmuir.6b00543) (hal-01415128) |
| David Duche, Ujwol Palanchoke, Luigi Terracciano, Florian-Xuan Dang, Lionel Patrone, Judikael Lerouzo, Teodor Silviu Balaban, Claude Alfonso, Ahmed Charai, O. Margeat, Jörg Ackermann, Cécile Gourgon, Jean-Jaques Simon, Ludovic Escoubas, Molecular Diodes for Optical Rectennas, NANOSTRUCTURED THIN FILMS IX, 2016, 9929 (10.1117/12.2237734) (hal-01435130) |
| Xavier Lefèvre, Fabrice Moggia, Olivier Segut, Yu-Pu Lin, Younal Ksari, Grégory Delafosse, Kacem Smaali, David Guérin, Vincent Derycke, Dominique Vuillaume, Stéphane Lenfant, Lionel Patrone, Bruno Jousselme, Influence of Molecular Organization on the Electrical Characteristics of π‑Conjugated Self-Assembled Monolayers, Journal of Physical Chemistry C, 2015, 119, pp.5703-5713 (10.1021/jp512991d) (hal-01228999) |
| V. Malytskyi, J.-J. Simon, Lionel Patrone, J.M. Raimundo, Thiophene-based push-pull chromophores for small molecule organic solar cells (SMOSCs), RSC Advances, 2015, 5, pp.354-397 (10.1039/C4RA11664J) (hal-01130036) |
| V. Malytskyi, J.-J. Simon, Lionel Patrone, J.M. Raimundo, Synthesis, self-assembly and characterization of a novel push-pull thiophene-based chromophore on gold surface, RSC Advances, 2015, 5, pp.26308-26315 (10.1039/C5RA02200B) (hal-01132858) |
| Lionel Patrone, Grégory Delafosse, Bruno Jousselme, Yu-Pu Lin, Younal Ksari, Mathieu Abel, Mathieu Koudia, Jean-Marc Themlin, Serge Palacin, Impact of $\pi$-conjugated self-assembled monolayer structure on their electrical properties, TNT2014, 2014 () (cea-02362750) |
| Alexandre Sangar, Alexandre Merlen, Philippe Torchio, Sylvain Vedraine, François Flory, Ludovic Escoubas, Lionel Patrone, Gregory Delafosse, V Chevallier, Eric Moyen, Margrit Hanbücken, Fabrication and characterization of large metallic nanodots arrays for organic thin film solar cells using anodic aluminum oxide templates, Solar Energy Materials and Solar Cells, 2013, 117, pp.657-662 (10.1016/j.solmat.2012.12.018) (hal-00911134) |
| Alexandre Merlen, Philippe Courmontagne, Alexandre Sangar, Philippe Torchio, Sylvain Vedraine, V Chevallier, Lionel Patrone, François Lagugné-Labarthet, Statistical Study of Metallic Nanostructures Prepared by Nanosphere Lithography, 6ièmes Journées Scientifiques du Centre de compétences en Nanosciences et Nanotechnologies (C'NANO-PACA 2013), 2013 () (hal-00911161) |
| Alexandre Merlen, Alexandre Sangar, Philippe Torchio, Sylvain Vedraine, V Chevallier, Philippe Courmontagne, Lionel Patrone, François Lagugné-Labarthet, Statistical Study of Metallic Nanostructures Prepared by Nanosphere Lithography, Surface Canada 2013 - Energy, Materials, Environment conference, London (CA), 2013 () (hal-00911168) |
| Dmitry A. Valyaev, Sylvain Clair, Lionel Patrone, Mathieu Abel, Louis Porte, Olivier Chuzel, Jean-Luc Parrain, Grafting a homogeneous transition metal catalyst onto a silicon AFM probe: a promising strategy for chemically constructive nanolithography, Chemical Science, 2013, 4, pp.2815-2821 (10.1039/c3sc50979f) (hal-00861617) |
| Gregory Delafosse, Alexandre Merlen, Sylvain Clair, Lionel Patrone, A Surface Enhanced Raman Spectroscopy study of aminothiophenol and aminothiophenol-C60 self-assembled monolayers: evolution of Raman modes with experimental parameters, The Journal of Chemical Physics, 2012, 136, pp.194704 (10.1063/1.4717720) (hal-01007241) |
| L. Porte, Mathieu Abel, P. Amsalem, F. Bocquet, C. Bocquet, V Chevallier, Sylvain Clair, G. Delafosse, S. Desbief, Virginie Gadenne, L. Giovanelli, Mathieu Koudia, Y. Ksari, C. Loppacher, Alexandre Merlen, Laurent Nony, O. Ourdjini, Lionel Patrone, R. Pawlak, J Romann, J Valmalette, J Themlin, Self-organised growth of molecular arrays at surfaces, International Journal of Nanotechnology, 2012, 9 (10.1504/IJNT.2012.045337) (hal-01896199) |
| S. Desbief, Lionel Patrone, D. Goguenheim, D. Vuillaume, Different types of phase separation in binary monolayers of long chain alkyltrichlorosilanes on silicon oxide, RSC Advances, 2012, 2, pp.3014-3024 (10.1039/C2RA01327D) (hal-00787367) |
| Alexandre Merlen, V Chevallier, J Valmalette, Lionel Patrone, Philippe Torchio, Sylvain Vedraine, Golam Moula, Surface enhanced spectroscopy with gold nanostructures on silicon and glass substrates, Surface Science : A Journal Devoted to the Physics and Chemistry of Interfaces, 2011, 605, pp.1214-1218 (10.1016/j.susc.2011.04.004) (hal-00911089) |
| S. Desbief, Lionel Patrone, D. Goguenheim, David Guérin, D. Vuillaume, Impact of chain length, temperature, and humidity on the growth of long alkyltrichlorosilane self-assembled monolayers, Physical Chemistry Chemical Physics, 2011, 13, pp.2870-2879 (10.1039/c0cp01382j) (hal-00572642) |
| Lionel Patrone, Jérémie Soullier, Pascal Martin, Role of S-Au labile bonding in stochastic switching of molecular conductance studied by STM, physica status solidi (b), 2010, 247, pp.1867-1870 (10.1002/pssb.200983829) (hal-03293608) |
| Alain Merlen, V Gadenne, J Romann, V Chevallier, Lionel Patrone, J Valmalette, Surface enhanced Raman spectroscopy of organic molecules deposited on gold sputtered substrates, Nanotechnology, 2009, 20, pp.215705 (10.1088/0957-4484/20/21/215705) (hal-03126122) |
| Wladimir Marine, N.M. Bulgakova, Lionel Patrone, Igor Ozerov, Insight into electronic mechanisms of nanosecond-laser ablation of silicon, Journal of Applied Physics, 2008, 103, pp.094902 (10.1063/1.2903527) (hal-00303806) |
| S. Desbief, Lionel Patrone, D. Goguenheim, D. Vuillaume, Nanostructured self-assembled monolayers of long-chain alkyltrichlorosilanes on Si, International Conference on Nano-structures Self-assembling, 2006 () (hal-00127135) |
| Lionel Patrone, S. Desbief, D. Goguenheim, D. Vuillaume, Growth of molecular nanostructures on Si using SAMs of long-chain alkytrichlorosilanes, Trends in NanoTechnologies, TNT2006, 2006 () (hal-00127136) |
| C. Trapes, L. Rouai, Lionel Patrone, Electrical Characterisation of Ultra-thin SAM Structures, ENS 2005, 2005, pp.55-57 () (hal-00166986) |
| S. Desbief, Lionel Patrone, D. Goguenheim, D. Vuillaume, Formation of nano-domains in SAMs of long chain alkyltrichlorosilanes deposited on silicon, 8th European Conference on Molecular Electronics, ECME8, 2005 () (hal-00125600) |
| Lionel Patrone, Serge Palacin, Jean-Philippe Bourgoin, Martinus H. V. Werts, Versatility of aqueous micellar solutions for self-assembled monolayers engineering, Langmuir, 2004, 20, pp.11577-11582 (10.1021/la048133b) (cea-01056938) |
| S. Desbief, Lionel Patrone, D. Goguenheim, D. Vuillaume, Formation of nano-domains in alkyltrichlorosilane self-assembled monolayers deposited on silicon : applications to molecular electronics, Ultimate Lithography and Nanodevice Engineering, 2004 () (hal-00140740) |
| Wladimir Marine, Nadezhda Bulgakova, Lionel Patrone, Igor Ozerov, Electronic mechanism of ion expulsion under UV nanosecond laser excitation of silicon: Experiment and modeling, Applied physics. A, Materials science & processing, 2004, 79, pp.771-774 (10.1007/s00339-004-2783-y) (hal-00001276) |
| Lionel Patrone, Serge Palacin, Julienne Charlier, Franck Armand, Jean-Philippe Bourgoin, Hao Tang, Sebastien Gauthier, Evidence of the Key Role of Metal-Molecule Bonding in Metal-Molecule-Metal Transport Experiments, Physical Review Letters, 2003, 91, pp.96802 (10.1103/PhysRevLett.91.096802) (cea-01056956) |
| Lionel Patrone, Serge Palacin, Jean-Philippe Bourgoin, Direct comparison of the electronic coupling efficiency of sulfur and selenium alligator clips for molecules adsorbed onto gold electrodes, Applied Surface Science, 2003, 121-213, pp.446-451 (10.1016/S0169-4332(03)00130-2) (cea-01056949) |
| Sébastien Decossas, Lionel Patrone, Anne-Marie Bonnot, Fabio Comin, Mickael Derivaz, André Barski, Joel Chevrier, Nanomanipulation by atomic force microscopy of carbon nanotubes on a nanostructured surface, Surface Science : A Journal Devoted to the Physics and Chemistry of Interfaces, 2003, 543, pp.57-62 (10.1016/S0039-6028(03)00919-1) (hal-00477221) |
| Lionel Patrone, Serge Palacin, Jean-Philippe Bourgoin, Jerome Lagoute, Tomaso Zambelli, Sebastien Gauthier, Direct comparison of the electronic coupling efficiency of Sulfur and Selenium anchoring groups for molecules adsorbed onto gold electrodes, Chemical Physics, 2002, 281, pp.325-332 (10.1016/S0301-0104(02)00373-7) (cea-01056952) |
| Lionel Patrone, Igor Ozerov, Marc Sentis, Wladimir Marine, Interaction des photons UV avec le silicium massif et nanocristallin, Journal de Physique IV Proceedings, 2001, 11, pp.121 (10.1051/jp4:2001738) (hal-00000835) |
| Igor Ozerov, Lionel Patrone, Marc Sentis, Wladimir Marine, Modification of Silicon Films and Nanostructures Deposition by Electric Field During UV Laser Ablation, E-MRS - IUMRS - ICEM 2000 Meeting (Symposium D – 'Photon-induced Material Processing'), 2000 () (hal-01897761) |
| Tatiana Itina, Lionel Patrone, W. Marine, M. Autric, Numerical analysis of TOF measurements in pulsed laser ablation, Applied physics. A, Materials science & processing, 1999, 69, pp.s59-s65 (10.1007/s003399900339) (ujm-00378610) |


